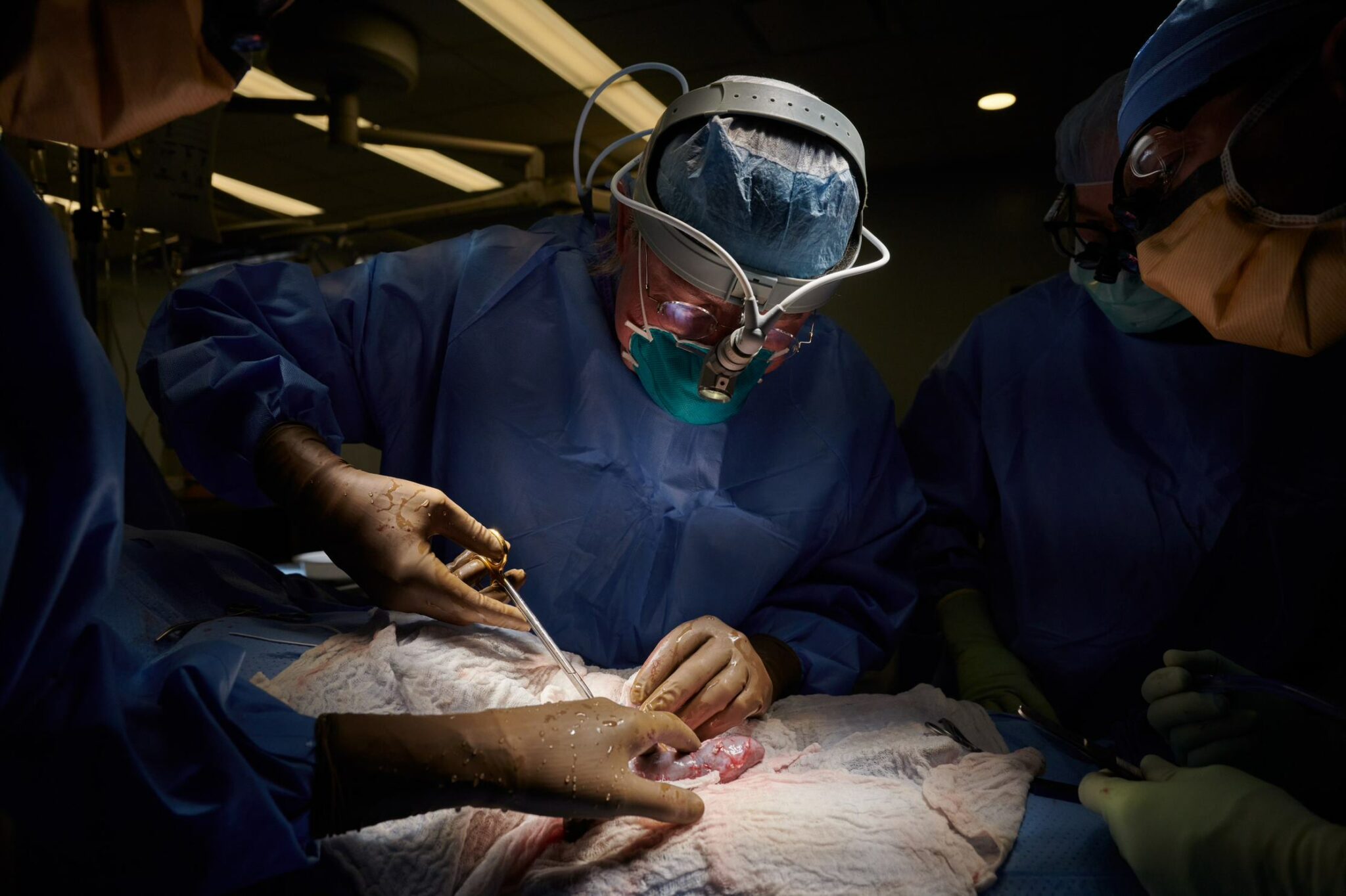
Another day, another xenotransplant, as United Therapeutics looks to beat competitors to sci-fi-esque breakthrough
Xenotransplantation is having a moment.
Last October, a team from NYU successfully transplanted a kidney from a pig into a brain-dead patient, although observers cast doubt on the importance of the experiment. Then, earlier this month, surgeons at the University of Maryland transplanted a pig heart into a dying human, who appears to still be stable.
Now, another group is planting a flag in the xenotransplantation field. Surgeons at the University of Alabama at Birmingham said Thursday they have achieved the first kidney transplant from a pig to a brain-dead patient, publishing their peer-reviewed findings online. The team, aiming to differentiate itself from the others through the genetic modifications used, is hoping there’s now enough research to soon begin clinical xenotransplantation studies.
Unlock this article instantly by becoming a free subscriber.
You’ll get access to free articles each month, plus you can customize what newsletters get delivered to your inbox each week, including breaking news.