At the center of a global tempest, Jiankui He defends editing the genes of 2 newborns
HONG KONG — Facing a storm of criticism from some of the medical world’s best known experts and under investigation by Chinese officials for producing the world’s first gene-edited babies in a self-funded experiment that has spurred headlines around the world, Jiankui He took to the center stage of a prominent scientific conference in Hong Kong to defend his work.
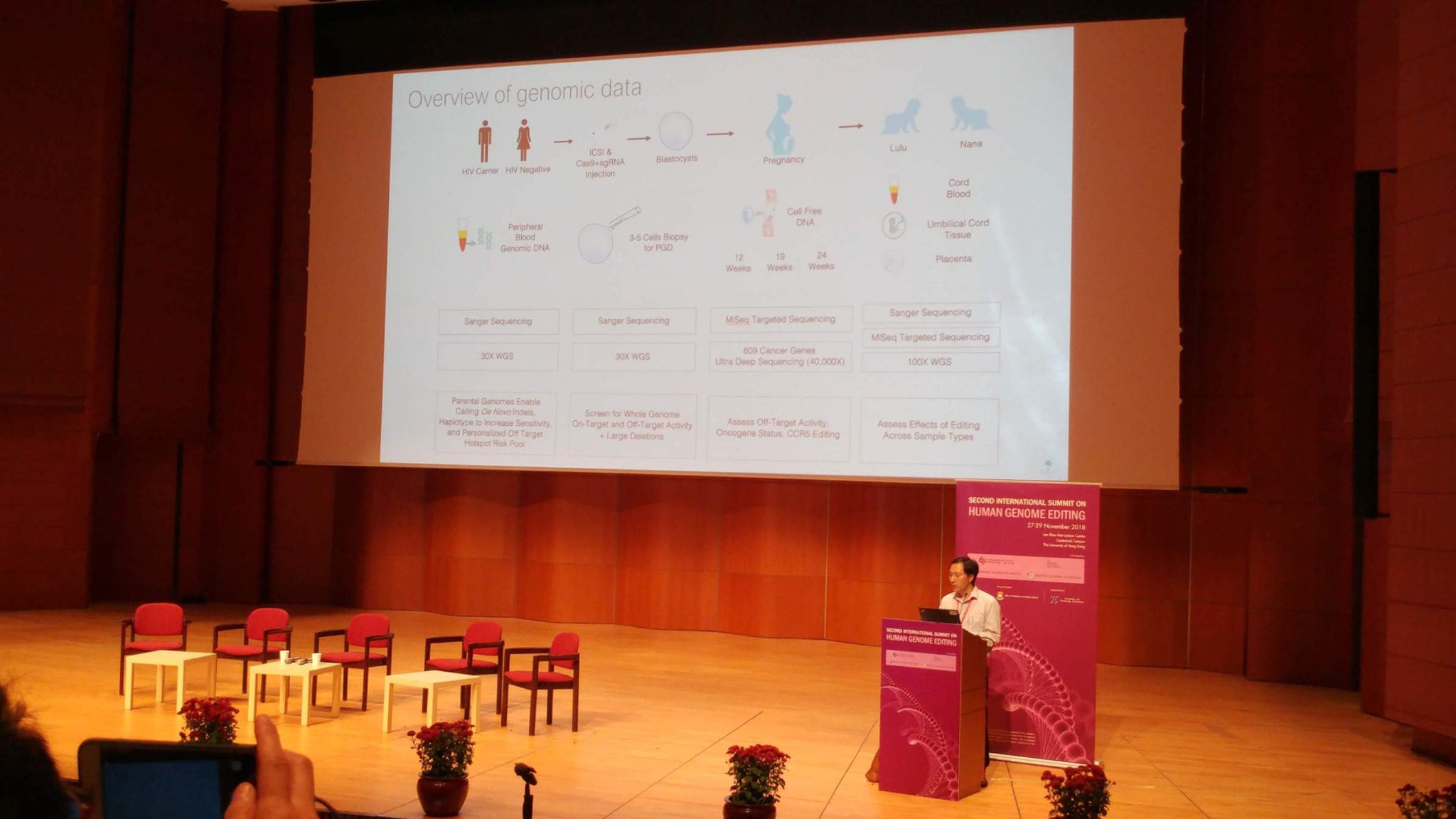
Calmly and clearly, He outlined his project using CRISPR to disable a key gene in order to confer immunity to HIV for the newborns — who were exposed to the threat by an HIV-positive parent — and offered an apology as the news that he had altered embryos “leaked unexpectedly” before he could present it at a scientific venue.
Unlock this article instantly by becoming a free subscriber.
You’ll get access to free articles each month, plus you can customize what newsletters get delivered to your inbox each week, including breaking news.