Cancer R&D stars Jim Allison, Tasuku Honjo awarded Nobel prize for immunotherapy discoveries
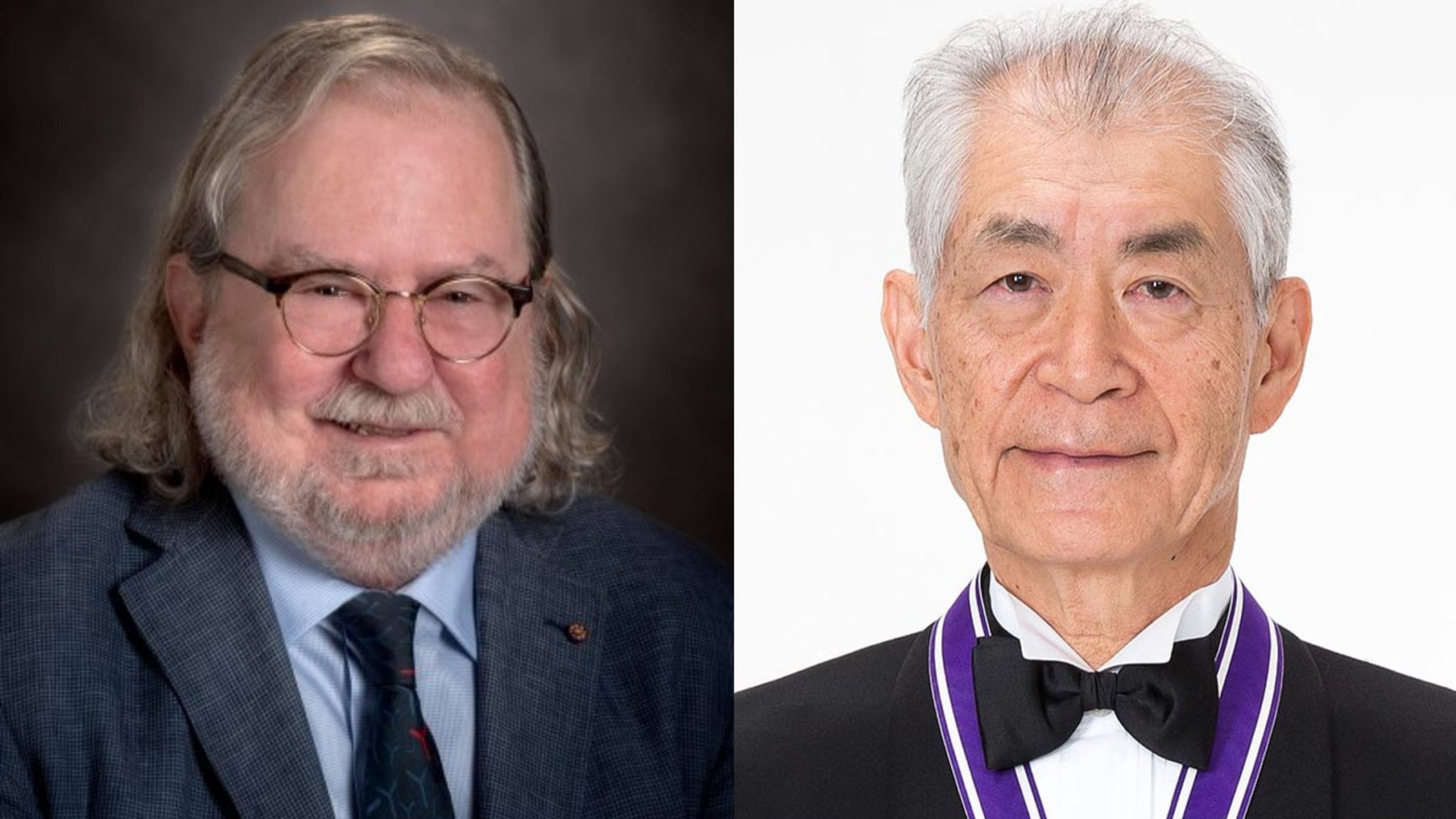
Immunotherapy pioneers James Allison and Tasuku Honjo has jointly won the 2018 Nobel prize in physiology and medicine “for their discovery of cancer therapy by inhibition of negative immune regulation.”
Allison, now a professor at MD Anderson and an affiliate of the Parker Institute, came to fame for his pioneering idea to unleash a T cell attack on cancer cells by blocking the protein CTLA-4. Honjo, a longtime faculty at Kyoto University, is credited with the discovery of PD-1 on the surface of T cells — which has been the basis of most checkpoint therapies currently on the market.
Unlock this article instantly by becoming a free subscriber.
You’ll get access to free articles each month, plus you can customize what newsletters get delivered to your inbox each week, including breaking news.