
Clinical trial adaptations made during the pandemic reveal an industry embracing change
For years, paper-based processes and individual point solutions dominated the clinical research landscape, and patient participation in clinical trials was largely an in-person engagement. But when the COVID-19 pandemic took a stronghold, traditional clinical trial methods emerged as inadequate, putting clinical trials and the life sciences industry at a crossroads. Practically overnight, the industry had to rapidly shift to decentralized clinical trial methods, while maintaining data quality and regulatory compliance.
Now it is evident that the methods utilized during the pandemic are likely to have a lasting impact on the future of clinical trials, according to a new research study conducted by Informa Pharma Intelligence and Oracle. Indeed, an overwhelming majority of survey respondents believe that new clinical trial methods are here to stay.
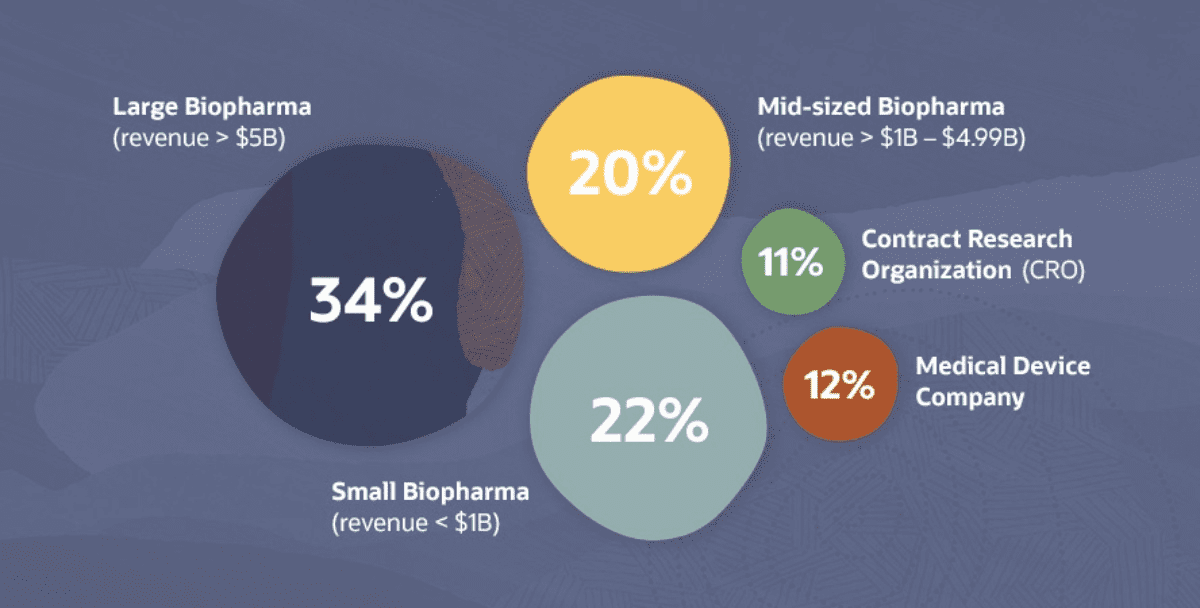
Out of a survey of 251 professionals from biopharmaceutical companies (76%), with the balance representing medical device companies and CROs, 97% of respondents who adopted new clinical trial methods during the pandemic revealed that their organization plans to continue using at least one of the new methods going forward.
“The industry had to move quickly to keep clinical trials afloat and this forced change has helped the industry understand when and how to implement these approaches to improve clinical research,” said Henry McNamara, senior vice president and general manager of Oracle Health Sciences. “Methods and technology that were being explored pre-pandemic have come front and center during the past 18 months.”
In last year’s industry survey conducted by Informa Pharma Intelligence and Oracle, 76% of respondents indicated that the COVID-19 pandemic had accelerated their adoption of decentralized clinical trial methods.
Decentralization leverages remote technology—such as wearable devices, sensors, mobile applications, and telehealth—to conduct clinical trial activities and data collection as close to the patient as possible, rather than at a study site. Patient data is entered into clinical trial management platforms, such as Oracle Health Sciences Clinical One. This flexibility not only delivers better results for the clinical trial, but it also makes participation easier for the patient. The survey revealed that 58% of respondents’ organizations plan to give patients the ability to choose how they participate in clinical trials going forward, which may lead to faster and larger trials, and ultimately, faster drugs to market.
Survey respondents said the decentralized, technology-based methods resulted in more timely data, improved flexibility for patients, increased speed, and higher patient participation and retention—all crucial components of a successful clinical trial. And the survey numbers bear this out: 82% of respondents who implemented new clinical trial methods during the pandemic reported seeing a positive impact on clinical trials overall, with 26% reporting a significantly positive impact.
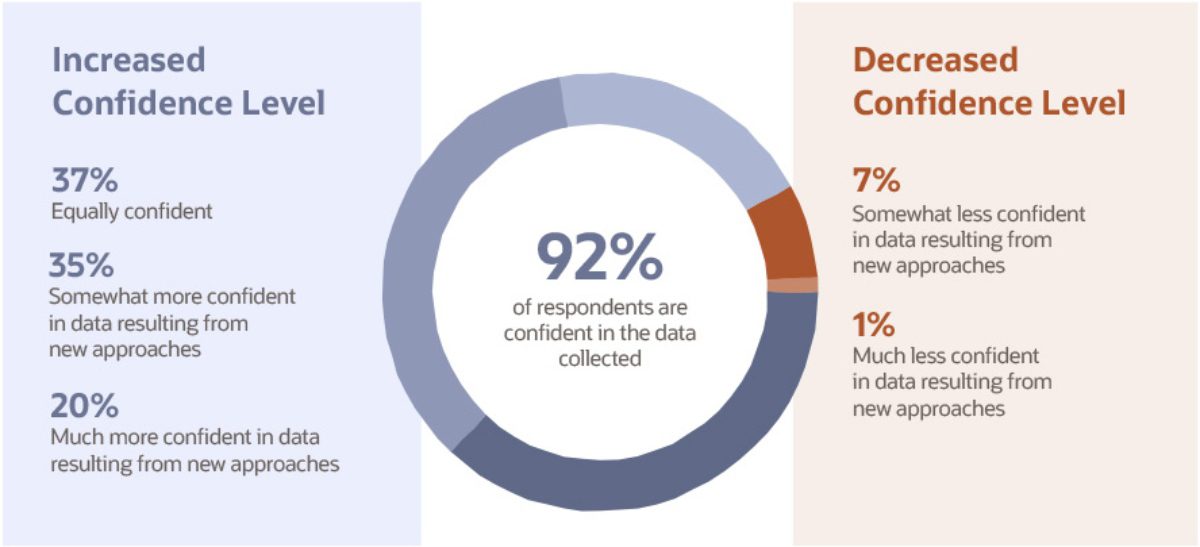
Decentralized clinical trials result in massive amounts of data, both in volume and variety. As such, it is not surprising that many of the positive impacts of clinical trial approaches newly implemented during the pandemic are related to data. According to the study, 92% of respondents who adopted new clinical trial methods reported that they were equally or more confident in the data collected from these methods, compared to data from traditional methods used before the pandemic.
Accurate and reliable data is essential in a clinical trial, as is the management of and access to confidential patient and operational data. Oracle’s Clinical One was designed to collect data from any source and allow faster adoption of new remote technologies. It lets a study team operationalize decentralized patient data capture and get a complete picture of a patient’s experience in a single place. Study teams can also collect and consider data from electronic health record systems and genomics data.
Most options on the market for collecting and storing clinical trial data are integrated systems, whereas Clinical One is a unified platform that brings together data from disparate sources and makes it available through a single access point. The idea is to provide clinical trial teams and sites with the ability to manage more patient data with greater clinical insight and less system complexity.
While the forced adoption of fit-for-purpose, hybrid, and decentralized models during the pandemic was unprecedented, it helped the industry understand when and how to implement these approaches and also highlighted the impact that technology and software can have on the ongoing advancement of clinical trials and drug development. This is reflected in results of this survey, with respondents expecting the continued use of these models in the post-pandemic world and giving patients the option to choose how they participate in clinical trials.
“As companies have adopted innovative approaches to clinical trials through the pandemic, we’ve proven the technology and software to support these changes in clinical trial research exists and will carry the industry forward,” said McNamara. “As clinical trials continue to evolve, study teams can rest assured that technology will not slow them down.”
To learn more about the study, you can read the full report here. Learn more about Oracle Health Sciences Clinical One here.