
How ClinicReady by Australian CRO Avance Clinical delivers preclinical project management and scientific and regulatory advice to get biotechs into clinic faster
Avance Clinical has formed a dedicated scientific and regulatory affairs service, ClinicReady by Avance Clinical, in response to increased demand from biotechs for preclinical study management and scientific and regulatory advice to take their products to first-in-human trials.
The highly regarded Avance Clinical scientific and regulatory team, which has been advising biotech clients on their drug development for more than 20 years, is now a dedicated ClinicReady service under the Avance Clinical banner.
 Yvonne Lungershausen, Avance Clinical’s CEO
Yvonne Lungershausen, Avance Clinical’s CEOAvance Clinical is an Australian owned Contract Research Organisation (CRO) that has been providing high-quality clinical research services to the local and international drug development industry for over 20 years. Avance Clinical specialises in working with biotechnology companies to execute early phase clinical trials.
“With our two decades of CRO experience we have become acutely aware of the importance of advising clients earlier in the development process, prior to them commencing preclinical safety and toxicology activities, so they conduct an appropriately balanced set of preclinical studies to get the right data required for approval of their first-in-human study in Australia,” said Yvonne Lungershausen, Avance Clinical’s CEO.
The ClinicReady Team
Avance Clinical’s ClinicReady team of scientific and medical affairs specialists comprise PhD qualified individuals with decades of experience in industry and academic research. They provide clients with scientific, regulatory and medical writing services, preparation of investigator brochures, clinical trial designs, study protocols, and patient information and consent forms as well as clinical trial data and clinical study reports.
 Ben Edwards, Avance Clinical’s Chief Strategy Officer
Ben Edwards, Avance Clinical’s Chief Strategy Officer“Many of our clients are biotechs looking to get investor support to progress their project to demonstration of early Proof of Concept (POC) which will enable them to access return on their investment through a licensing deal to Pharma. This scenario also ensures high probability of success in bringing promising treatments to the market and available to the patient population,” commented Ben Edwards, Avance Clinical’s Chief Strategy Officer.
Avance Clinical has created the ClinicReady by Avance Clinical range of services in response to demand from biotechs for expertise in preclinical study management and scientific and regulatory advice to take their products to first-in-human trials.
 Jorgen Mould PhD,
Jorgen Mould PhD,BSc (Hons) Avance Clinical’s Scientific Affairs Specialist
“In order to expand our range of services to cater for the preclinical research management needs of small biotechnology enterprises and assist them in bringing their products to clinical trials we have recently announced the addition of Dr Jorgen Mould to our ClinicReady scientific and medical affairs team,” commented Yvonne Lungershausen.
Dr Jorgen Mould joined Avance Clinical in the role of Scientific Affairs Specialist. He has over 20 years of experience in the medical industry specialising in drug discovery and clinical development. With depth of therapeutic expertise in neurobiology and inflammation, Jorgen has managed the progress of several projects through preclinical development to progression to first-in-human trials and is uniquely positioned to assist Avance Clinical’s clients with management of regulatory approval and bringing promising preclinical products to the clinic.
“Having spent several years taking products to the clinic in the biotechnology sector, I am really looking forward to the opportunity of sharing my experience with Avance Clinical’s clients in a way that expedites access to the clinical trial testing for their preclinical assets,” commented Dr Jorgen Mould.
From Discovery to Proof of Concept with Avance Clinical
The ClinicReady range of services constitutes a natural progression in Avance Clinical’s role as the premier provider of early drug development support to the biotechnology sector. The company recognises the major challenges faced by small biotechnology companies, particularly start-up companies who seek to drive their novel therapeutics to demonstration of safety and preliminary clinical Proof of Concept. Start-up companies often need to leverage development of their products without the time and cost burden associated with hiring staff and accessing appropriate expertise. With ClinicReady, Avance Clinical’s team can act as a surrogate drug development department for start-up companies who are looking to benefit from a virtual model of operationalisation and execution from the early stages of drug candidate discovery to demonstration of preliminary clinical POC.
With ClinicReady, Avance Clinical’s team can act as a surrogate drug development department for start-up companies
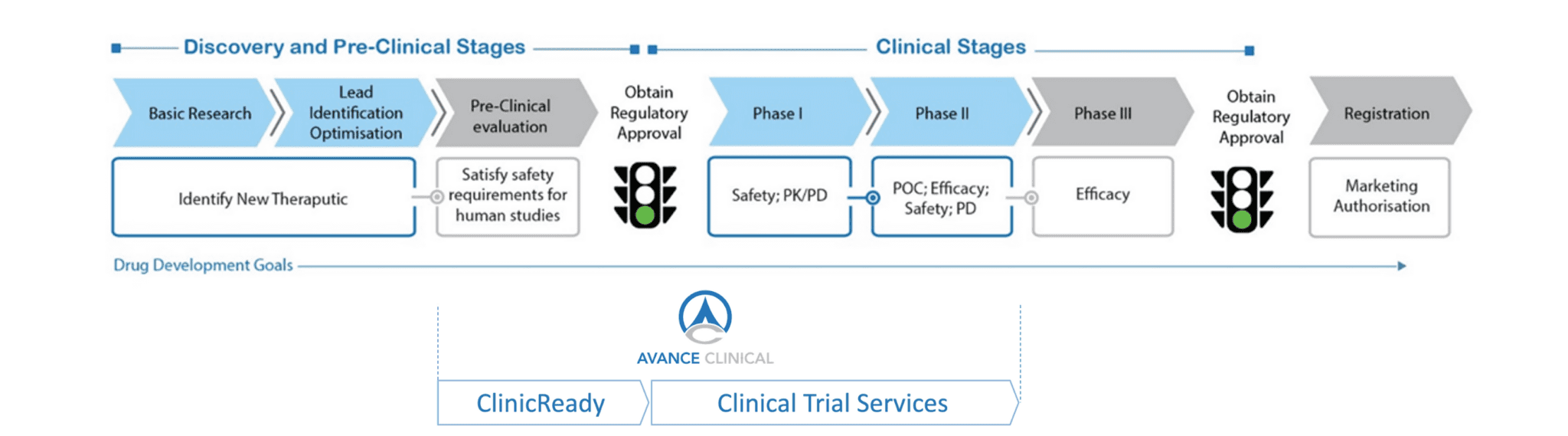
ClinicReady enables Avance Clinical to further differentiate itself from other clinical CROs by firmly embedding its range of services in the early drug development space incorporating both preclinical and clinical aspects. Avance Clinical’s model is of a translational CRO helping biotech companies to progress laboratory research discoveries to early clinical proof of concept and effectively translating preclinical molecules to promising clinical therapeutics.
ClinicReady by Avance offers the following services:
- Preparation of Drug Development Plans (from discovery to phase II proof of Concept)
- Program gap analysis with particular emphasis on preclinical study plans
- Preclinical vendor selection and management
- Scientific Advice on translational aspects with emphasis on attaining evidence of pharmacological effect early in clinical development
- Therapeutic Area Advice with particular emphasis on product differentiation and positioning within existing and evolving therapeutic paradigms
- Chemistry Manufacturing Controls (CMC) and Investigational product management Advice
- Preparation of Investigator’s Brochure
- Regulatory agency submission and Human Research Ethics approval support
The Australian Landscape – Preclinical Studies to Enable Drug Candidate Progress to Clinical Investigation
Promising drug candidates showing early proof of concept in animal models of human disease need to be further evaluated for safety before they can progress into human trials. First-in-human trials have the greatest element of risk in relation to drug safety. Prior to evaluating novel therapeutics in humans, it is critical to obtain appropriate preclinical data which informs the potential risks and defines dose levels and dosing regimens to determine an acceptable risk/benefit profile.
In relation to preclinical data requirements, the Therapeutic Goods Administration (TGA) in Australia recommends a number of ICH and EMA guideline documents for the Australian equivalent of IRBs, the Human Research Ethics Committees (HREC), to take into consideration when reviewing applications for the conduct of clinical trials in Australia.
The primary objectives of preclinical studies should aim to:
- Establish a proof of concept to provide a rationale for the target therapeutic indication
- Obtain information required to guide dosing in human studies
- Identify target organs/tissues or physiological processes which are likely to drive adverse events
- Inform on safety parameters that should be monitored in the human trial
- Establish a risk/benefit profile of the intended therapy.
As human safety is the key concern in early phase trials, pivotal preclinical safety and toxicology studies should comply with Good Laboratory Practice (GLP) standards. A typical set of preclinical studies which would capture the necessary information to define the dosing parameters and risk/benefit profile of a drug candidate prior to entering the clinic includes (but is not limited to) the following:
Primary Pharmacology (non-GLP)
- In vitro data demonstrating the activity of the drug on the therapeutic target (cell-based assays, enzyme assays, target binding)
- In vivo data showing activity in disease relevant animal models
- Pharmacokinetic data enabling efficacy-to-exposure correlation
Safety Pharmacology
- In vitro off Target screen (protein class, common safety receptor panel) (non-GLP)
- In vitro hERG inhibition, to exclude cardiac liability (GLP)
- Central nervous system safety pharmacology, in vivo (GLP)
- Respiratory system safety pharmacology, in vivo (GLP)
- Cardiovascular system safety pharmacology, in vivo (GLP)
Toxicology
- 7-day dose range finding toxicity study in male and female rats (non-GLP)
- 28-day repeat dose toxicity and toxicokinetic study in male and female rats (GLP)
- 7-day dose range finding toxicity study in male and female dogs or other non-rodent species (non-GLP)
- 28-day repeat dose toxicity and toxicokinetic study in male and female dogs or other non-rodent species (GLP)
Metabolism
- Plasma protein binding in rat, dog, and human plasma (non-GLP)
- Microsomal stability in rat, dog, and human microsomes (non-GLP)
- Hepatocyte stability in rat, dog, and human hepatocytes (non-GLP)
- CYP450 inhibition/induction (non-GLP)
Genotoxicity
- Bacterial reverse mutation assay (GLP)
- In vitro mammalian chromosomal aberration assay in human peripheral blood lymphocytes (GLP)
- Mammalian micronucleus assay in rats with flow cytometric analysis in peripheral blood reticulocytes (GLP)
It is important to note that the preclinical GLP toxicology studies in two species (e.g. rat, dog) are of central significance to defining the key parameters of a First-in-Human trial. The preclinical toxicology studies should be adequate to identify and characterize potential toxic effects of the drug candidate to allow investigators to conclude that it is reasonably safe to proceed to clinical investigation. Aspects to be considered in designing animal toxicology studies include the choice of relevant animal species and strain, dosing schedule and route of administration, as well as timing of drug administration and evaluation of endpoints (e.g. sampling for clinical chemistry). The toxicology study duration and number of doses of test drug administered to animals should be equivalent to or exceed that used for the clinical study.
Gaining Approval to Conduct Clinical Trials in Australia
There are two alternative schemes to gaining approval to conduct a clinical trial in Australia.
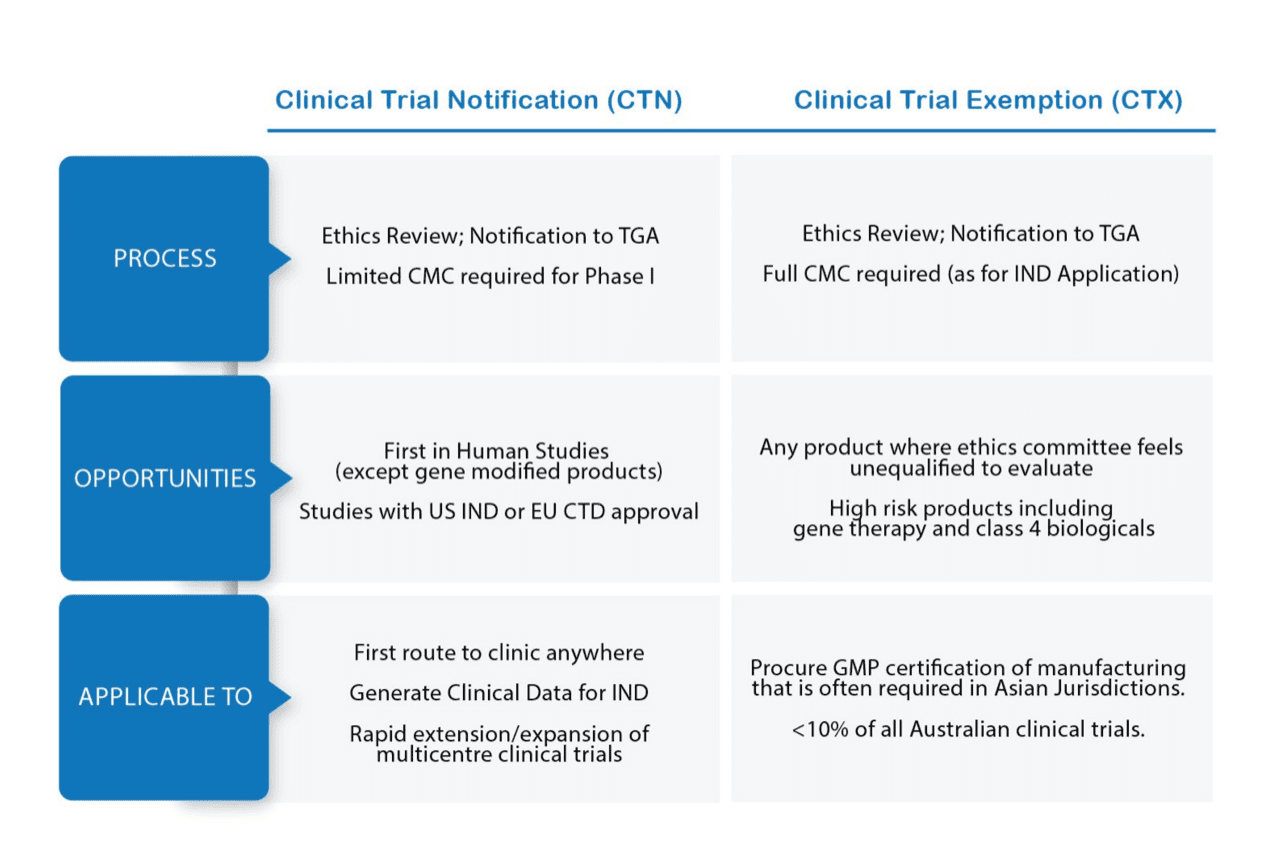
In Australia, the majority (>90%) of clinical trials can be submitted via the CTN scheme whereby preclinical data summarised in an Investigator Brochure are reviewed by an HREC who then notifies the TGA of the outcome. This provides an opportunity to conduct early phase trials in Australia whilst preparing documentation required for an IND submission to the FDA
Watch our ClinicReady webinar here: https://www.biospectrumasia.com/avance-clinical