Innovation by Disruption: Breaking Silos Between R&D and Commercial Teams to Advance New Treatments for Patients
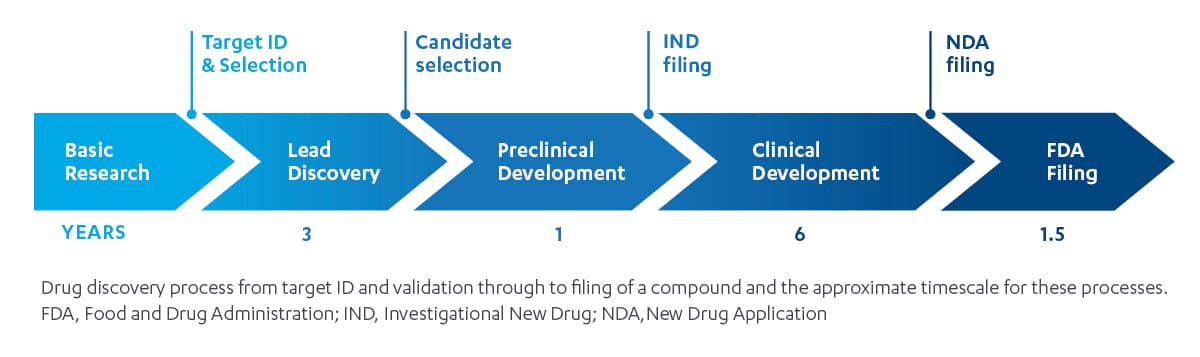
The clinical development pathway: a highly curated, step-by-step, functional process, has remained largely unchanged for decades across the biopharmaceutical industry. It is a well-worn operation that can result in 15 years between drug concept and commercialization, and a high attrition rate.1 A key challenge to innovation and the acceleration of improved medicines for patients is this long-standing model of siloed research and development (R&D) and commercial teams within an organization. At Janssen, we’ve changed our paradigm and demonstrated that more integrated decision-making throughout the process can enhance the value of what we as an industry deliver for patients, for the healthcare system, and for all stakeholders.
The industry standard functional approach: the domino effect
The insights and decision-making of this siloed paradigm operates like a line of dominos: the process moves only in one direction, with each domino only touching the dominos that are adjacent to it. The “line” starts with a discovery team that identifies promising compounds from a pool of candidates they advance to become new molecular entities (NMEs). Translational medicine is next, preparing NMEs for human trials with pharmacokinetic and pharmacodynamic modeling, and pre-formulation. Then, an early development team proceeds with Phase 1 and Phase 2a human trials, followed by late development, the dose finding Phase 2b, and confirmatory pivotal Phase 3 trials it conducts to finalize dosing, endpoint inclusion and hierarchy for regulatory filing and labeling strategy. Finally, the commercial team launches the new therapy in market.
This model presents a key challenge: deep thinking, insights and decisions happen primarily within each function, as each of the specialized teams described includes experts who may have limited expertise outside their fields.
Like the line of dominos, impact – in the form of insights — are transferred only from left to right, and sometimes with contradictory incentives, or not at all. Once a domino goes down, it’s difficult to go back.
Janssen Immunology and the de-siloed approach: Newton’s cradle
At Janssen Immunology, we’re developing and delivering transformational medical innovations for patients suffering from a range of devastating, difficult to treat, chronic immune-mediated diseases, driven by a relentless dissatisfaction with the status quo.
We employ a fundamentally different model in how drug discovery and development functional experts from diverse disciplines work together and how our teams operate to accelerate innovative medicines. Our model operates more like a Newtown’s cradle than dominos. Each functional team, like the metal balls of Newton’s cradle transfer energy – or expertise and insights – through the balls from one end, then back again throughout the development process.2 For example, future-back market insights inform prioritization of biochemical properties as early as hit to lead, and translational medicine insights inform lifecycle planning. We operate in a strategic decision-making framework and corresponding operational model with minimal organizational boundaries between early target assessment and late life cycle management. This promotes insights and expertise to flow continuously across R&D and commercial functions. It is a close partnership between our R&D and commercial functions, and a fully integrated approach to drug development, that enables us to harness insights across the value chain.
There are five core tenets of the Janssen Immunology drug development model:
- Shared joint R&D and commercial leadership commitment to a de-siloed, end-to-end operating model: all functions are committed to structuring their teams around this model.
- Singular shared vision and mission from discovery through commercialization.
- Joint governance model: a disciplined approach is critically important to operating with an integrated portfolio lens. We don’t just question whether a program, or program progression can technically happen. Rather, we challenge programs to identify which have the greatest potential for substantially advancing treatment paradigms vs. all available therapies at time of approval. We believe opportunity costs matter as much as direct program costs. We deploy deep insights into actionable science and unmet need to quickly inform ‘killer questions’ and to implement stringent prioritization criteria in our portfolio decisions.
- Talent: among senior leaders, our aim is to hire “learners” versus “knowers.” Learners are willing to challenge the status quo and to gather learnings from areas outside their own expertise. Knowers, considered “experts” because they’ve been in the job for a long time, tend to base decisions squarely on their own experience.
- Clear accountability with “brain currency” orientation: because there are broad inputs to any decision, ultimate accountability for each decision must be crystal clear. When accountable leaders make a decision, they either view the “brains” around them as impediments or as currency to work toward a solution.
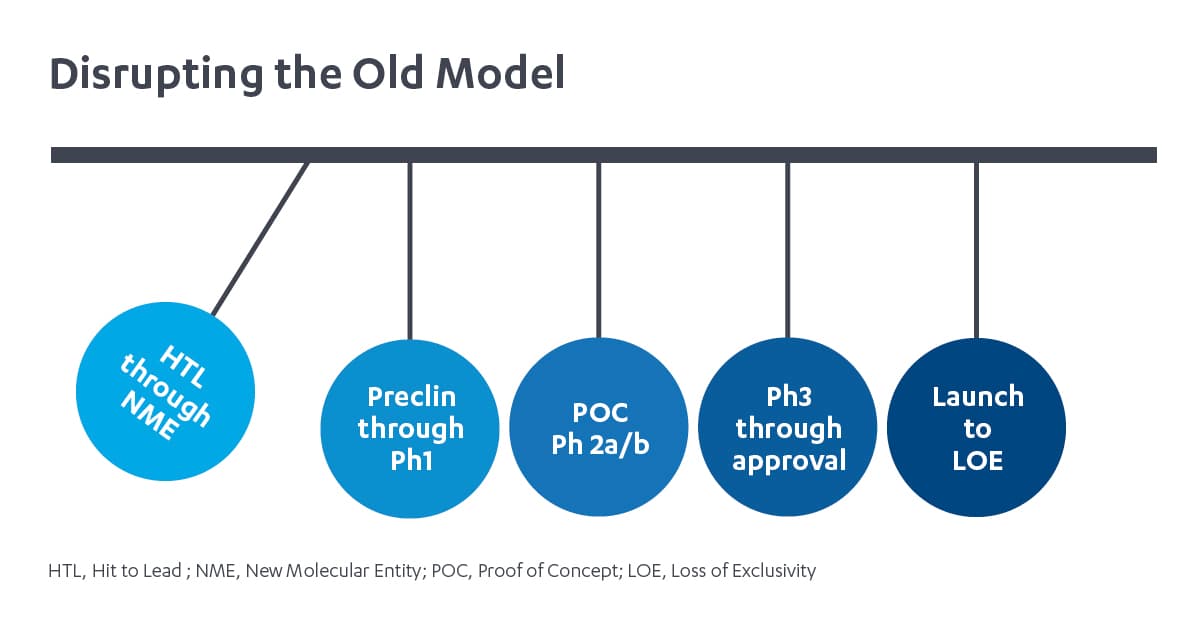
In our de-siloed model, each functional group still makes decisions, but they are made with insights into implications up and down the drug development process. The result is a clearer and more aligned prioritization, smarter trial design, and more differentiated assets. This has made Janssen Immunology uniquely poised to develop medicines for conditions across disease areas: those that are rare, such as hemolytic disease of the fetus and newborn, and prevalent, as evidenced by our long-standing commitment to deliver treatments for people with rheumatoid arthritis.
How do these differing functional approaches affect drug development success rates?
Since 2005, across the industry, immunology research has seen about a 31% success rate in Phase 2 trials, and an 81% success rate in Phase 3.3 Comparatively, Janssen Immunology has seen a 45% success rate and 92% success rate in Phase 2 and Phase 3 trials over the same time frame.3 We’ve also achieved more than two decades of continuous growth and innovation on behalf of patients, with five internally developed, marketed products, and more than 31 indication approvals, 18 of which are first-in-class, and four blockbuster therapies that have helped to redefine standard of care in chronic immune-mediated diseases.
By breaking silos throughout the discovery, development, and commercialization process, we have revealed efficiencies and produced results for patients who need them. This has led us to scientific discoveries, new data insights, and treatments that were considered impossible 10 years ago. Patients are waiting.
References:
- Hughes JP, Rees S, Kalindjian SB, Philpott KL. Principles of early drug discovery. Br J Pharmacol. 2011;162(6):1239-1249. doi: 10.1111/j.1476-5381.2010.01127.x
- Schulz, C. HowStuffWorks: How Newton’s Cradles Work. Updated: Apr 8, 2021. https://science.howstuffworks.com/innovation/inventions/newtons-cradle.htm#pt2
- CMR Global R&D Performance Metrics Programme. CMR International is a Clarivate Analytics business
Authors:
 David M. Lee, M.D., Ph.D.,
David M. Lee, M.D., Ph.D., Global Therapeutic Area Head, Immunology
 Teri Lawver,
Teri Lawver, Worldwide Vice President, Immunology