Optimizing Cell and Gene Therapy Development and Production: How Technology Providers Like Corning Life Sciences are Spurring Innovation
Remarkable advances in cell and gene therapy over the last decade offer unprecedented therapeutic promise and bring new hope for many patients facing diseases once thought incurable. However, for cell and gene therapies to reach their full potential, researchers, manufacturers, life science companies, and academics will need to work together to solve the significant challenges facing the industry.
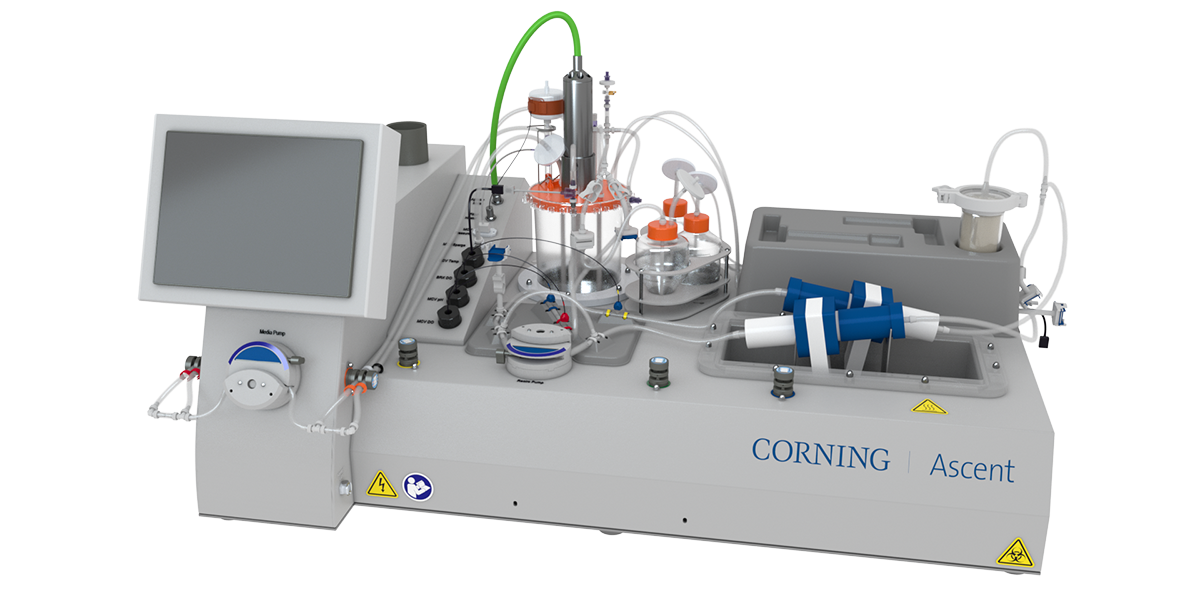
While time to market and production cost head the list of priority concerns for nearly every industry, several common contributing factors, top-of-mind for users, include scalability, automation, and cell type flexibility. Corning Life Sciences has a long history of advancing adherent platform technologies and working with industry innovators, such as Cellipont Bioservices, to help enable efficient scale-up strategies in cell and gene therapy production. Most recently, Corning launched the Corning® AscentTM Fixed Bed Bioreactor system, the latest result of customer collaborations and technological innovations in this area.
As a leading life sciences company, Corning is dedicated to accelerating innovation and solving customer challenges. From early research to production, there are aspects of the cell culture process that can greatly influence time to market and cost. Three of the most pressing challenges are outlined here:
- Scalability—processes should be able to move from the lab to production while minimizing technology transfer and re-development steps as much as possible. Having to move from adherent platforms to suspension to achieve desired scale can require additional development and optimization steps, as well as time and costs.
- Automation—systems should be as automated as possible. Lack of monitoring capabilities and automated processes can result in greater overhead costs via increased staff and the opportunity for human error.
- Cell type flexibility—customers working across advanced therapeutic modalities require systems that give them flexibility to work with various cell types and applications (e.g., stem cells, cells for viral vector production, etc.). Lack of flexibility drives up costs as companies must invest in and maintain several production platforms.
Aiming to address scalability, automation, and cell type flexibility challenges from the earliest inceptions of projects is key to improving the likelihood of later success and efficiency, especially when selecting platform technologies for development of advanced therapies. Corning has been at the forefront of adherent cell culture platforms since the introduction of the well-known Corning High Yield Performance (HYPER) technology more than a decade ago, and continues that commitment to increasing cell density with the Ascent FBR system, which includes the in-market Process Development platform and forthcoming Pilot and Production systems.
Recently, fixed-bed reactor technologies have marked an important step in addressing the most limiting hurdles of adherent platforms. The Ascent FBR system is an intensified, high-yield cell culture platform designed to enable linear scalability—from process development and seed train to production scale. It is also fully automated, including key process parameter monitoring, bringing one of the most significant benefits of suspension platforms to adherent cell culture. Finally, the Ascent FBR system’s viable cell harvest capability enables the system to support multiple cell types and applications, including both ‘cell as a product’ (e.g., stem cell therapies) and ‘cell as a producer’ (e.g., viral vectors) production processes.

Zara Melkoumian, Ph.D., Business Technology Director, and the Ascent FBR system
“When our data revealed we could have a significant improvement in AAV vector yield when using the Ascent FBR System, we got quite excited. The validation from our beta customers confirms the tremendous value and potential of the platform to support critical breakthroughs.”
—Zara Melkoumian, Ph.D., Business Technology Director, Corning Life Sciences
By enabling customers to centralize their workflows in a single platform, the Ascent FBR system is helping to standardize workflows that make the translation to production easier. These benefits together can offer a cumulative process advantage when choosing the Ascent FBR system and can potentially drive innovation forward at a faster pace, for more diverse applications, and at a lower cost.
Cellipont Bioservices (formerly Performance Cell Manufacturing), a company known for its expertise in cell therapy development and manufacturing, has recently collaborated with Corning to assess new technologies. Cellipont helps their clients incorporate best-in-class capabilities, facilitating scale up from benchtop, moving through clinical studies, and in the near future, supporting commercialization with hopes of expanding access to life saving medicines to patients across the world. Most recently, Cellipont and Corning applications scientists have been working closely to evaluate the Ascent FBR platform as an emerging technology capable of meeting the need for scalable biomanufacturing.
As a master of many trades, including the manufacturing of stem cells, chimeric antigen receptor T cells, dendritic cell therapies, and plasma derived therapy products, Cellipont has an eye for innovative, flexible systems.
“We are always looking to present new technological solutions to our clients as we position ourselves as partners in their scientific efforts. We see a lot great of potential in the Ascent FBR system, including efficient scale up and cost savings both from a consumables perspective and a hands-on perspective—all in a small footprint. The fact that it is a closed system is also critically important.”
—Carolyn Wrightson, Chief Technology Officer, Cellipont Bioservices

Corning works closely with customers like Cellipont to understand the unique goals, processes, and unmet needs of those using their products, and to provide customized solutions. Field applications scientists work hand-in-hand with labs to overcome challenges and to help ensure that users are easily and effectively incorporating new technologies, from HYPERFlasks® to the Ascent FBR platform, into their workflows.
With deep industry knowledge and more than 100 years of experience, Corning remains committed to furthering customers’ capabilities in adherent cell culture. By bringing to market novel technologies that can offer an efficient scale-up strategy from lab to production, Corning is working closely with developers and manufacturers to help them bring therapies to market more quickly and efficiently. Cell and gene therapy is at a critical inflection point, and through such cross-industry collaboration, it is poised to realize therapeutic realities the world has only imagined.
