ZS Perspective: 3 Predictions on the Future of Cell & Gene Therapies
The field of cell and gene therapies (C>s) has seen a renaissance, with first generation commercial therapies such as Kymriah, Yescarta, and Luxturna laying the groundwork for an incoming wave of potentially transformative C>s that aim to address diverse disease areas. With this renaissance comes several potential opportunities, of which we discuss three predictions below.
Allogenic Natural Killer (NK) Cells have the potential to displace current Cell Therapies in oncology if proven durable.
Despite being early in development, Allogenic NKs are proving to be an attractive new treatment paradigm in oncology. The question of durability of response with allogenic therapies is still an unknown. Fate Therapeutics’ recent phase 1 data for FT516 showed relatively quicker relapses vs already approved autologous CAR-Ts. However, other manufacturers, like Allogene for their allogenic CAR-T therapy ALLO-501A, are exploring novel lymphodepletion approaches to improve persistence of allogenic cells. Nevertheless, allogenic NKs demonstrate a strong value proposition relative to their T cell counterparts due to comparable response rates (so far) combined with the added advantage of a significantly safer AE profile. Specifically, little to no risk of graft versus host disease (GvHD), cytotoxic release syndrome (CRS), and neurotoxicity (NT) have been seen so far with allogenic NK cells (Fig. 1). In addition, being able to harness an allogenic cell source gives way to operational advantages as “off-the-shelf” products provide improved turnaround time (TAT), scalability, and potentially reduced cost. NKs are currently in development for a variety of overlapping hematological indications with chimeric antigen receptor T cells (CAR-Ts) today, and the question remains to what extent they will disrupt the current cell therapy landscape. Click for more details.
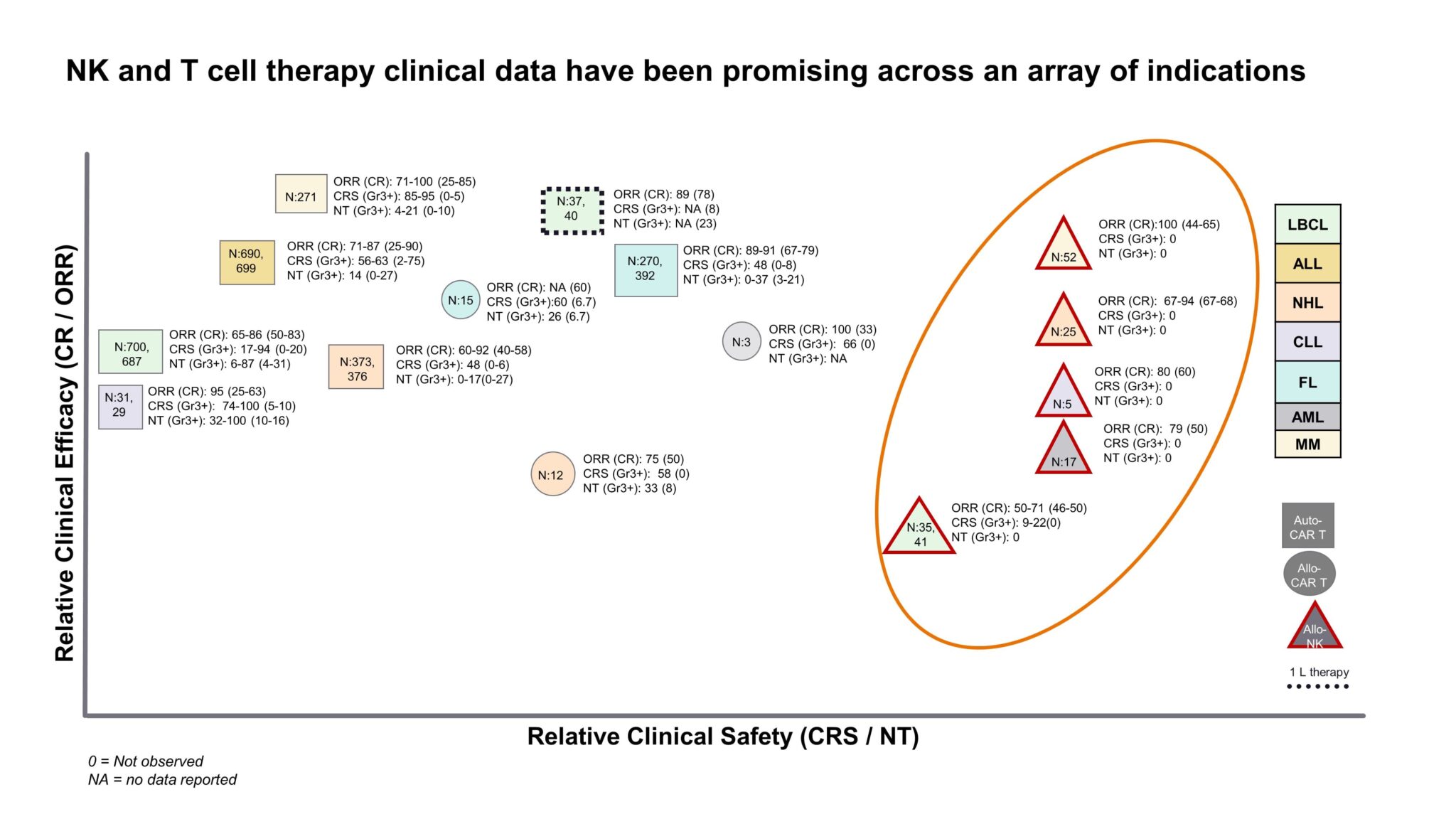
Figure 1: Efficacy and safety data for Autologous vs Allogenic cell therapies in development. Sample n sizes are provided for each point as N:x [1].
In-vivo gene therapies (vs cell-based therapies) could be the more prominent Cell & Gene technology in solid cancers.
An increasing number of in-vivo gene therapies are in clinical trials for solid cancers (Fig. 2). Gene therapies are poised to make deep inroads into solid tumor treatments with several unique mechanisms of action (MOAs) by which in-vivo gene therapies can target cancer ranging from activating apoptosis and recruiting immune cells as well as direct oncolytic effects. Although many of the gene therapies in solid cancer work in a more tangential way to tumor killing, usually recruiting other cancer killing cells, cell-based therapies are also facing efficacy struggles in overcoming the immunosuppressive tumor microenvironment (TME) in solid cancers. In-vivo gene therapies are better situated to handle the TME and are advantageous vs autologous cell therapies as an “off-the-shelf” option. While still early in development, the breadth of both the pipeline and possible MOAs make in-vivo gene therapies an attractive potential technology type for solid cancers in the future.
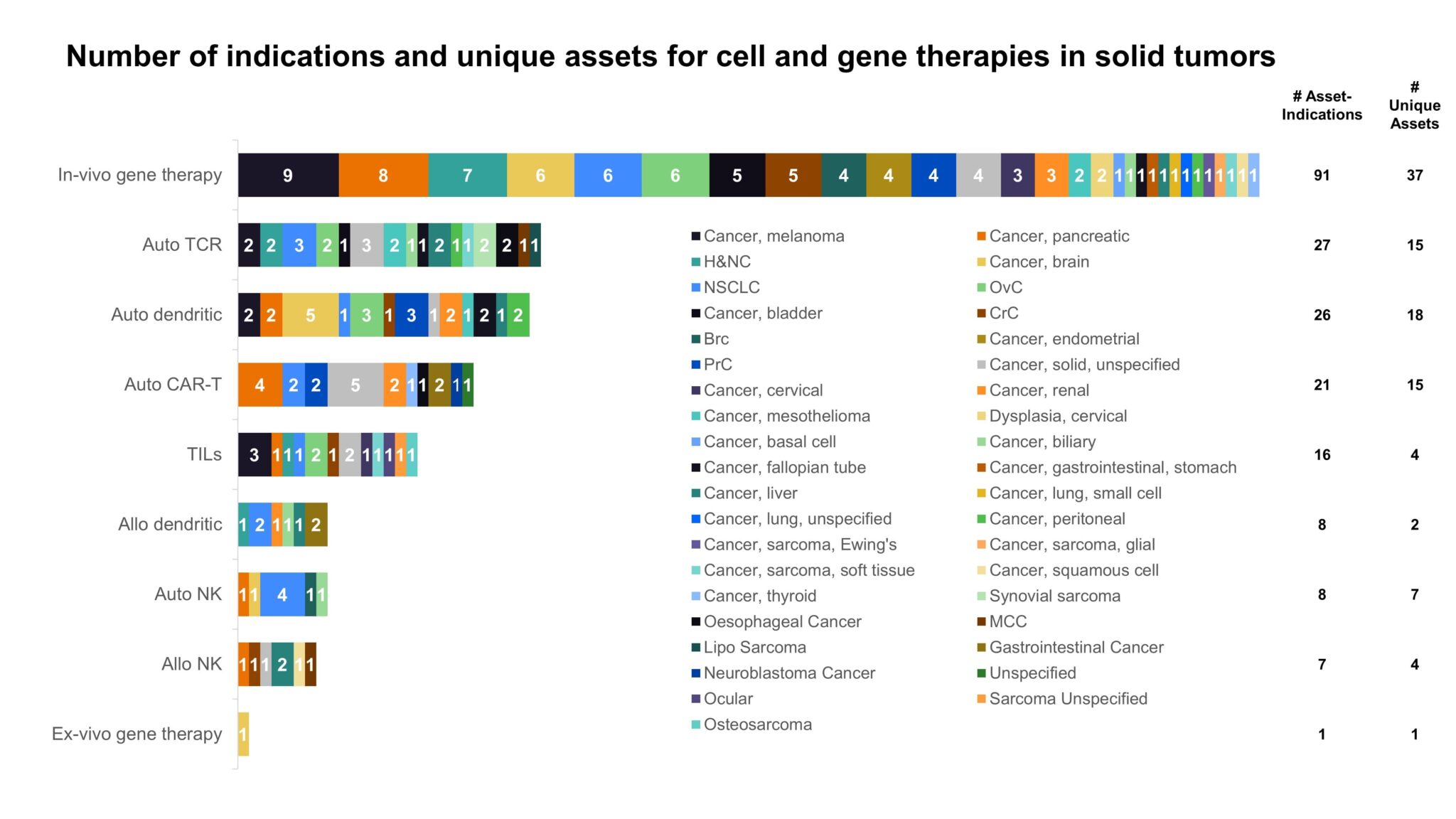
Figure 2: Ph II+ C>s by technologies across solid tumors with the number of unique assets by therapy type [1].
While the first wave of C>s are single agents, the future may be in combination.
It comes as no surprise that manufacturers are beginning to consider combinatorial approaches, leveraging the strengths of various technologies to provide revolutionary outcomes in high unmet need disease areas. Checkpoint inhibitor and CAR-T combinations are already in development while many other cell and gene therapy approaches are underway that can also be complementary in function (Fig. 3). However, the benefits need to be weighed against the risks as combining these potent therapies could exacerbate potentially fatal AEs, like CRS and NT. The pursuit of novel C> combinations is likely to continue the growing trend of identifying subsets of patients who are more likely to respond or at greater risk for severe AEs to better inform treatment decisions.
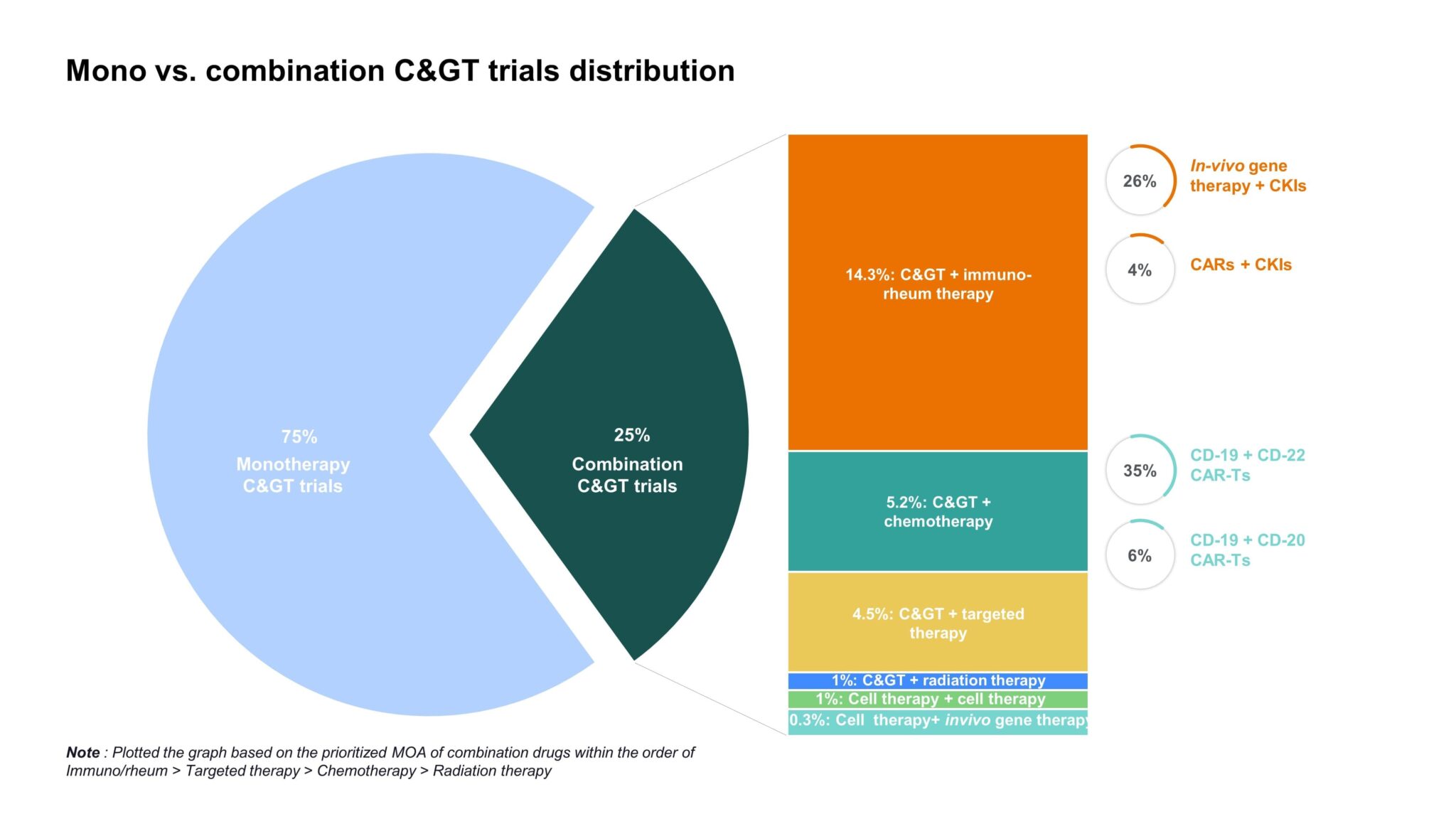
Figure 3: Overview of monotherapy vs. combination C> trials to date, illustrating that approximately 30% of cell and gene therapies are being studied in combination with various therapies, including immuno-rheum therapies, chemotherapy, targeted therapy, radiation therapy, in-vivo gene therapy, and other cell therapies [1].
The C> landscape is rapidly evolving
In just a short period from March 2021 to November 2021 we have observed a substantial fluctuation in assets entering / exiting clinical trials. 73 cell (61) and gene (12) therapy trials have paused or been terminated while 68 new cell (62) and gene (6) trials have been identified. Navigating this ever-changing C> landscape requires a comprehensive understanding of C> and how the field will continue to change from manufacturing, supply chain logistic, to pricing strategies and access issues. Here at ZS we have a team of experts who seek to comprehensively understand the complex C> landscape and these predictions are an excerpt from our broader e-book, read more here.
About ZS
ZS Emerging Biotech and Pharma Space: ZS is the leader in “first launch”, partnering with 200+ unique pre- and early-commercial biopharma companies on 1,000+ projects in just the last 5 years. ZS has a robust understanding of common challenges of development-stage emerging pharma, which consists of a growing number of C> manufacturers. With deep experience in areas such as portfolio and pipeline strategy, business development and licensing, pipeline asset strategy and launch strategy and readiness, ZS can help ensure that you realize the full potential of your pipeline and achieve commercial success beyond approval. Click here to stay up-to-date on ZS’s “First Launch” thought leadership and here to find out more about our Pipeline & Launch Strategy capabilities.
Cell and Gene Therapy Space: ZS has been a part of every US cell & gene therapy launch to date and most launches in the EU. In 2021, we partnered with 35+ manufacturers on 200+ projects in both the clinical and commercial settings. ZS’s C> space focuses on Discovery, Development and Delivery to help manufacturers jumpstart their C> portfolio, improve their manufacturing and sourcing strategy, develop end-to-end data strategies, and craft successful commercial, launch, access and expansion strategies – all while looking ahead to the rapidly evolving market space. ZS can help you prepare for a successful launch, especially in oncology and rare disease indications where we see the most C> development today. Find out more on ZS.com here.
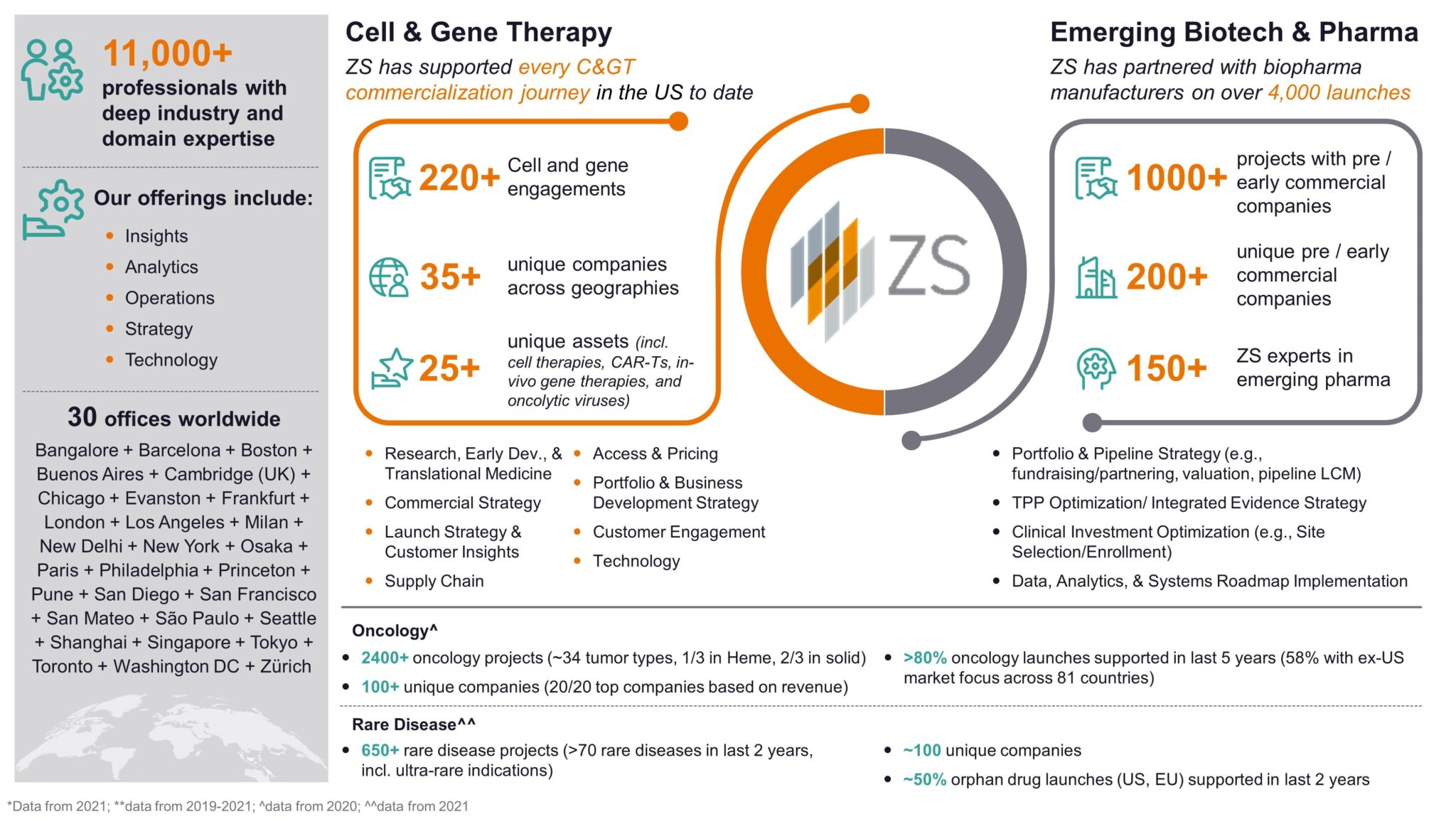
Figure 4: ZS is the leader in “first launch”, partnering with Emerging Biopharma and Cell & Gene Therapy companies on early strategy and planning through to execution phases.
References:
[1] List of references can be found in the full e-book or upon request.