Hairpins and Scissors - Delivering a Non-Gene Edited Allogeneic CAR T Cell Therapy for the Masses
Overview
The licensing of two Chimeric Antigen Receptor (CAR) T cell therapies for the treatment of B cell malignancies underscores the potential of cell based immune therapy to deliver impressive durable clinical responses¹. These products are autologous in nature which involves collecting immune cells from the patient that are used to manufacture the CAR T cells. Once produced, these CAR T cells are then reinfused as the clinical product to the patient. However, there are significant challenges to autologous therapy, including product production time (which currently takes weeks) during which the patient’s disease may progress, and the highly variable quality of the starting material, which can result in manufacturing failures.
Allogeneic CAR T cell therapy, an off the shelf approach that can be administered when required, is the ideal solution. This approach generates cells from a healthy donor to form a bank of CAR T cells that can be used as needed. The key challenge of allogeneic CAR T is overcoming a toxicity associated with the recognition of healthy patient tissues by the allogeneic CAR T cells. This is mediated by the T cell Receptor (TCR). Disrupting the TCR underpins all current allogeneic CAR T strategies².
Hairpins and Scissors
Currently, gene editing technologies used to generate allogeneic CAR T are at an early stage of clinical development. The different gene editing approaches are all based on cutting the genome within one of the genes that encode the TCR, which permanently reduces expression of the entire TCR complex. Whilst an elegant approach, this scissor strategy has been challenging to move into clinical testing due to potential product safety concerns – mainly ensuring the absence of ‘off-target’ genome cutting during gene editing³.
Alternatively, targeting gene expression at the mRNA level does not involve cutting the genome and avoids jeopardizing genome integrity. To deliver this mRNA ‘editing’, Celyad Oncology employs short hairpin RNA (shRNA), a method used over several decades to knockdown gene expression⁴. The approach involves using an shRNA that has a complementary sequence to that of the target gene. In other words, a targeted shRNA can specifically reduce the level of a desired protein such as the TCR complex by interfering with mRNA and not by cutting the genome⁵.
Central to this is the All-in-One vector approach. In one step, a single reagent (vector) introduced into healthy donor T cells results in the simultaneous production of all elements in the T cell that can re-direct the T cell against tumor (the CAR), eliminate the TCR (shRNA) and provide a handle where the modified cells can be enriched in manufacturing (the marker).
shRNA in an Allogeneic CAR T Cell Platform
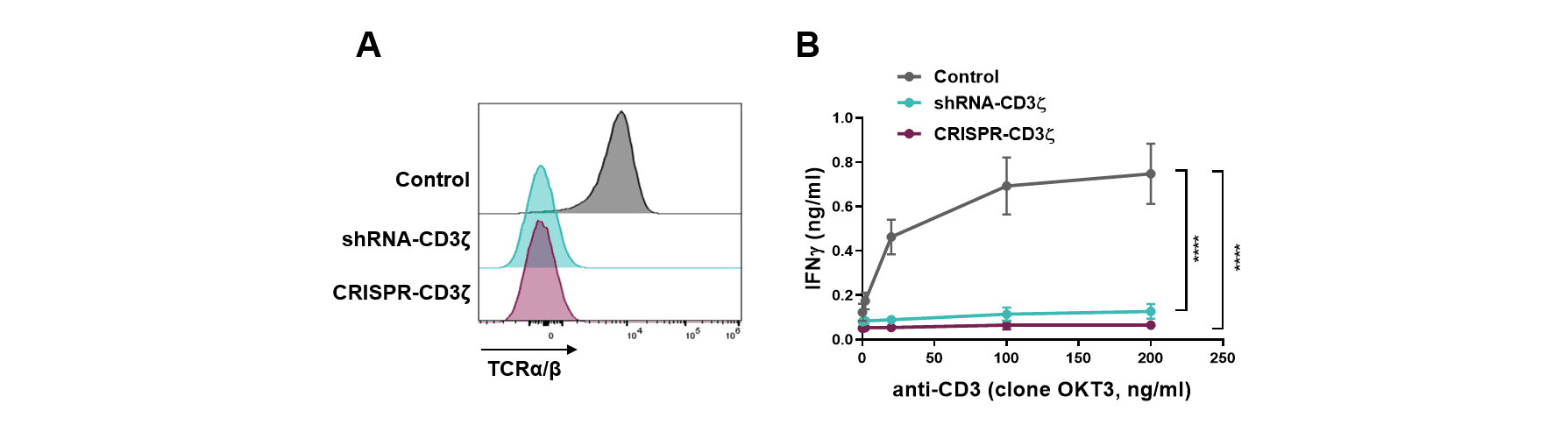
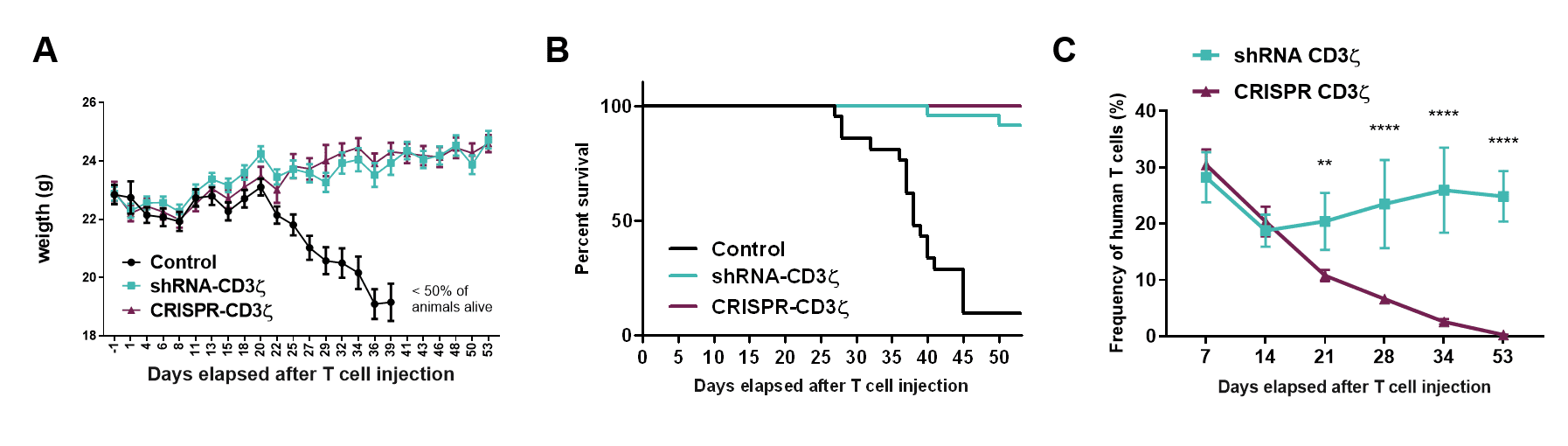
The CD3 zeta subunit provides the main signaling power to the TCR that enables activation and engagement of the T cells killing ability. Through the selection of an optimal shRNA and process development, targeting CD3 zeta results in durable high-level knockdown of the TCR on primary T cells to a level equivalent to that seen if the CD3 zeta gene was gene edited (Figure 1A). Functionally, this correlates with an inability of these cells to respond to a mitogenic stimulus (aka TCR driven T cell activation; Figure 1B) and a corresponding absence of toxicity when these cells are infused into the gold standard in vivo test model (Figure 2A, B). Interestingly, the persistence of the shRNA targeted T cells was much longer than that of the CRISPR-Cas9 gene edited equivalent cells (Figure 2C). The mechanism is still being investigated but clearly shows that T cells expressing a single shRNA against CD3 zeta can form a platform suitable for allogeneic CAR T cell therapy.
Figure 1: (A) Surface expression of TCR as assessed by detection of the TCRαβ subunit by flow cytometry, and (B) IFN-γ secretion in the supernatant upon 24-hour culture with increasing concentration of anti-CD3 antibody as TCR mitogenic stimulus, of T cells transduced with control vector (Control), transduced with a vector containing shRNA targeting CD3 zeta (shRNA-CD3 zeta) or transfected with CRISPR targeting the CD247 gene that encodes CD3 zeta (CRISPR-CD3 zeta).
Click on the image to see the full-sized version
Figure 2: (A) Weight kinetics, (B) Kaplan-Meier survival curves, and (C) T cell engraftment in NSG mice (n=5 per group) injected with 20×10⁶ T cells transduced with control vector (Control), transduced with a vector containing shRNA targeting (shRNA-CD3 zeta) or transfected with CRISPR targeting the CD247 gene that encodes CD3 zeta (CRISPR-CD3 zeta), 1 day after receiving 1.44 Gy total body irradiation.
Click on the image to see the full-sized version
CYAD-211; The First shRNA-Based Non-Gene Edited Allogeneic CAR T cell therapy
Celyad Oncology’s first clinical candidate employing shRNA technology in an allogeneic context is CYAD-211, an anti-BCMA therapy. The three key elements of CYAD-211 are:
- A second generation CAR that employs a BCMA-specific engager
- A single shRNA that targets CD3 zeta
- A marker gene that allows direct enrichment of the engineered allogeneic CAR T cells, as well as non-modified cells to be removed in a single manufacturing step
Pre-clinical studies confirmed that T cells engrafted with a BCMA CAR co-expressing the CD3 zeta targeting shRNA exhibited robust anti-tumor activity in vitro and in vivo with no evidence of toxicity (Figure 3). Importantly, the speed of taking this non-gene edited approach from initial concept to clinical trial testing in around two years is a testament to the potential of the technology from a regulatory context
Figure 3: Kaplan-Meier survival curves of NSG mice (n=5 per group) intravenously injected with vehicle or 10⁷ T cells transduced with a control vector (mock) or with a vector containing a BCMA-targeting CAR and a shRNA targeting CD3 zeta, 6 days following intravenous injection of 5×10⁶ KMS-11 cancer cells.
Click on the image to see the full-sized version
The Future of Non-Gene Edited Allogeneic Approaches
CYAD-211’s future in-human trials aim to prove the potential for a single shRNA allogeneic approach. However, the small size of the transgene and potential to express multiple shRNAs (multiplexing) will enable future clinical candidates to modulate the expression level of multiple gene products, thereby generating T cells with desired phenotypic and functional properties. Importantly, as compared to gene editing, the level of gene knockdown can also be controlled through the choice of the specific shRNA.
This is particularly important where the knockout of a protein may cause issues of viability, such as with essential kinases, or through the knockout of a protein like HLA class 1 which would sensitize the CAR T cell to natural killer cell elimination.
In addition, shRNA multiplexing provides a means to generate an optimal therapeutic T cell phenotype with a strong control on one of the major raw material costs since all these elements are maintained within a single vector. Engineering multiple knockdowns using current gene editing approaches will require an increasing number of clinical grade reagents, which the All-in-One vector approach avoids.
Overall, shRNA provides a potent platform with the power to control the level of single or multiple genes to optimize allogeneic CAR T cell products.
References
- June CH, O’Connor R S, Kawalekar OU, Ghassemi S & Milone MC. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018)
- Depil S, Duchateau P, Grupp SA, Mufti G & Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat. Rev. Drug Discov. 19, 185–199 (2020)
- Kosicki M, Tomberg K & Bradley A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 36, 765–771 (2018)
- Barata P, Sood AK & Hong DS. RNA-targeted therapeutics in cancer clinical trials: Current status and future directions. Cancer Treat. Rev. 50, 35-47 (2016)
- Giering JC, Grimm D, Storm TA & Kay MA. Expression of shRNA from a tissue-specific pol II promoter is an effective and safe RNAi therapeutic. Mol. Ther. 16, 1630-1636 (2008)