
New York-based partnership sets the standard for expanding clinical trial access for underserved and diverse populations
For decades, groundbreaking medical research has remained out of reach for many who need it most, leaving underserved communities with limited opportunities to participate. While numerous efforts have been made in recent years to address this issue, many have been fragmented and have failed to address the systemic barriers that limit access to clinical trials. The significance of this issue is underscored by the FDA’s June 2024 draft guidance, “Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Populations in Clinical Studies.”1 Setting aside recent controversy surrounding the document itself 2, the guidance highlights that the problem exists, still in 2025.
A recent New York-based partnership between Pratia, a global leader in clinical research, and Essen Health Care, a trusted healthcare provider in the Bronx, is taking the lead in addressing this issue by integrating clinical trials directly into community healthcare.
There is a lot of work to be done in the Bronx
With a population of 1.4 million, the Bronx faces exceptionally high rates of preventable conditions. Diabetes-related hospitalizations are nearly double the New York City average, while 39% of the Bronx residents have high blood pressure compared to 28% of all NYC residents—just two of many concerning examples.
Access to medical innovation should not be determined by geography or socioeconomic status. Yet, for many Bronx residents, participation in clinical trials—and with it, access to free health screenings and cutting-edge therapies—has been limited by several barriers: (1) mistrust, often rooted in past injustices in medical history and fueled by a lack of information and discomfort with the research process; (2) logistical challenges, such as time and resource constraints; and (3) limited understanding and awareness of available clinical trial opportunities 3.
Pratia powered by Essen – a case study
For the clinical trial operating model to be successful, it needs to address all of the above barriers simultaneously. This means meeting patients where they are—both physically and emotionally—building trust and working within the systems they are familiar with. It’s about minimizing the steps a patient undertakes to participate in a clinical trial. This approach is at the heart of Pratia’s vision; to transform the clinical research landscape by integrating innovative treatments into everyday patient care, making clinical trials a standard of care option. Rather than imposing a standardized, one-size-fits-all clinical site model, Pratia adapts its operating models to fit local contexts, ensuring that clinical trials become a seamless part of the healthcare journey.
This model has already proven successful in seven other countries where Pratia operates, utilizing both dedicated sites (e.g., Pratia Sirius in Spain) and integrated site models (e.g., the acquisition of CRC in Czechia and Slovakia).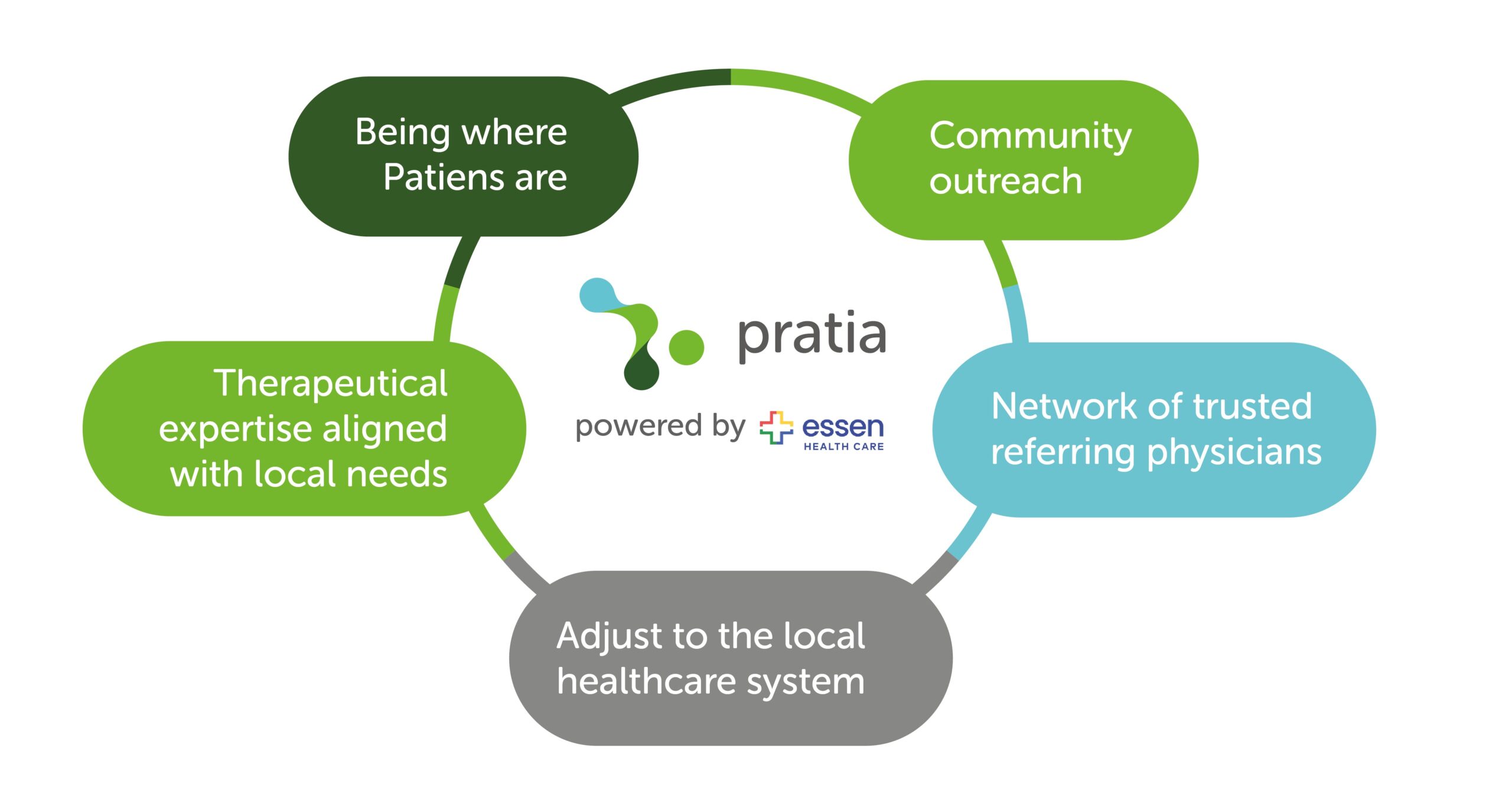
By embedding clinical trials into the local healthcare network, the partnership removes key barriers and brings advanced treatment options to the historically underserved community. The collaboration is built on five fundamental pillars:
- Being where patients are: Essen Healthcare operates over 45 clinics across the Bronx, directly within the communities that need expanded healthcare options the most. Beyond clinic-based care, Essen Health Care takes an extra step by offering Essen House Calls services, ensuring that even homebound Patients can have access to clinical trials by eliminating transportation and mobility challenges that often prevent participation in research.
- Therapeutic expertise aligned with local needs: As mentioned above, the Bronx has disproportionately high rates of chronic conditions across multiple therapeutic areas, including metabolic disorders, cardiology, pulmonology, and mental health—areas that align directly with Pratia’s research expertise. The partnership will include trials that address these pressing health concerns, ensuring that the studies conducted are both relevant and beneficial to the local population.
- Community outreach and education: Community engagement is an integral part of recruitment and retention of underrepresented groups4. This partnership prioritizes patient education, with Essen Health Care leveraging its deep roots in the Bronx to lead community outreach efforts. By providing clear, accessible information about clinical research, the initiative aims to foster trust and empower patients to make informed decisions about their healthcare.
- A network of trusted referring physicians: Studies show that physicians are the most trusted source of information for Patients considering clinical trials5. Essen Health Care clinicians’, who have long-standing relationships with their Patients, play a critical role in providing guidance for trial participation. With the right tools and -guidance from Pratia, they can introduce clinical trial opportunities as a natural extension of care, reinforcing trust and encouraging more patients—especially from minority populations—to enroll.
- Adjusting to the local healthcare system: Rather than imposing a rigid, disconnected clinical trial model, Pratia follows its mission to increase access to innovative treatments across diverse patient populations by seamlessly adapting to local needs, making clinical trials a natural extension of standard medical care. This flexibility enables different models depending on community requirements—whether through standalone dedicated research clinics, embedded oncology units within hospitals, or, as in the Bronx, establishing strong partnerships with local healthcare providers to expand research accessibility and patient engagement.
Why inclusion in clinical trials matters
Clinical trials shape the future of medicine, but their impact is directly related to the diversity of the participants involved. When research fails to include all racial, ethnic, and socioeconomic groups, the resulting treatments may not work equally well for everyone. The FDA has reinforced the urgency of addressing these gaps, pushing for more inclusive study populations. Pratia, through its partnership with Essen, is turning this vision into reality—embedding research into local healthcare networks, removing barriers to participation, and ensuring that groundbreaking therapies reach those who have historically been left behind. This is more than just expanding access; it’s about building a future where medical innovation truly serves everyone.
Pratia is part of the Humaneva Group.
Follow Pratia on LinkedIn to stay up to date.
References:
1Office of the Commissioner. (2024, June 26). Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Populations in Clinical Studies. U.S. Food And Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diversity-action-plans-improve-enrollment-participants-underrepresented-populations-clinical-studies
2Grossi, G. (2025, February 6). FDA quietly removes draft guidance on diversity in clinical trials following executive order on DEI. AJMC. https://www.ajmc.com/view/fda-quietly-removes-draft-guidance-on-diversity-in-clinical-trials-following-executive-order-on-dei
3Clark, L. T., Watkins, L., Piña, I. L., Elmer, M., Akinboboye, O., Gorham, M., Jamerson, B., McCullough, C., Pierre, C., Polis, A. B., Puckrein, G., & Regnante, J. M. (2019). Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Current Problems in Cardiology, 44(5), 148-172. https://doi.org/10.1016/j.cpcardiol.2018.11.002
4Kelsey, M. D., Patrick-Lake, B., Abdulai, R., Broedl, U. C., Brown, A., Cohn, E., Curtis, L. H., Komelasky, C., Mbagwu, M., Mensah, G. A., Mentz, R. J., Nyaku, A., Omokaro, S. O., Sewards, J., Whitlock, K., Zhang, X., & Bloomfield, G. S. (2022). Inclusion and diversity in clinical trials: Actionable steps to drive lasting change. Contemporary Clinical Trials, 116, 106740. https://doi.org/10.1016/j.cct.2022.106740
5Clark, L. T., Watkins, L., Piña, I. L., Elmer, M., Akinboboye, O., Gorham, M., Jamerson, B., McCullough, C., Pierre, C., Polis, A. B., Puckrein, G., & Regnante, J. M. (2019). Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Current Problems in Cardiology, 44(5), 148-172. https://doi.org/10.1016/j.cpcardiol.2018.11.002
Contributing author:
 Mona Alqam,
Mona Alqam,MD, Country Head,
Pratia USA