
How Innovative Technologies and a Dedicated Site Network is Driving Patient Recruitment for Biotech Clinical Trials in Australia
Australia’s CRO for biotechs, Avance Clinical, deep dives into the patient recruitment landscape identifying the mechanisms and processes that deliver exceptional patient recruitment for early phase trials in Australia.
Avance Clinical also offers models for later phase pivots to large patient populations in Central and Eastern Europe and the US via exclusive collaborations with more than 3,500 sites. Learn more here.
In Australia, Site Initiation Visit (SIV) and Study Start can be achieved in 5 – 6 Weeks, plus there is up to a 43.5% refund on clinical trial spend.
 Yvonne Lungershausen, CEO, Avance Clinical
Yvonne Lungershausen, CEO, Avance ClinicalAvance Clinical is the Australian owned full-service biotech CRO that has been providing high-quality clinical research services across all phases to the US, APAC, and EU drug development industry for over 20 years.
“Our team has extensive therapeutic area knowledge and expertise and has conducted drug trials with large molecules, small molecules, gene therapies and devices providing high-quality clinical research services fit for global regulatory standards,” said Yvonne Lungershausen, Avance Clinical’s CEO
“We offer end-to-end in-house clinical research services including drug development consultation, medical writing, clinical project management, trial monitoring, pharmacovigilance, data management, statistical and pharmacokinetic services, CDISC and external and internal auditing.”

All of the news, delivered with full-text to your inbox. For professionals discovering, developing, and marketing biopharmaceutical drugs.
Patient Recruitment Landscape
 Andy Hu, Corporate Account Manager at Pharma Intelligence, Informa
Andy Hu, Corporate Account Manager at Pharma Intelligence, InformaPatient recruitment success is fundamental to the success of clinical trials.
More than 50% of Avance Clinical’s trials involve patients seeking to support the development of new therapies for their conditions. As a result the company has built significant expertise in patient recruitment across a range of therapeutic areas. This includes utilizing world class market intelligence solutions, development of strong investigator site relationships and a range of recruitment vendors utilizing innovative technologies to increase the accuracy of recruitment rates and maximise patient recruitment during trial conduct.
Importantly, patient recruitment costs are covered by the 43.5% rebate incentive offered by the Australian Government.
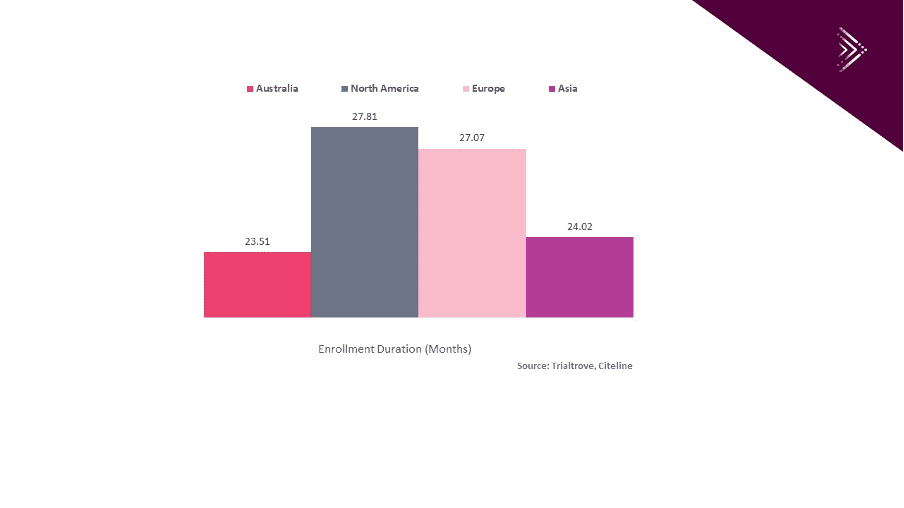
For example, Andy Hu, Corporate Account Manager at Pharma Intelligence, Informa reported that “according to Trialtrove, Citeline, from January 1st, 2010 to December 31st, 2019, Australian sites recruit patients faster for oncology trials than sites in North America, Europe and Asia.” As shown in Graph 1 below the average recruitment duration in Australia is 23.51 months for Oncology trials in Australia vs 27.81 months and 27.07 months in North America and Europe respectively.
Avance Clinical’s access to recruitment benchmark data utilizing technology such as world leading market intelligence solution Trialtrove, Citeline as well as our strong Investigator and site relationships allow us to accurately plan for on time study delivery.
Avance Clinical Recruitment Partners
Proven partners for this vital service
Avance Clinical will recommend and engage a comprehensive recruitment solution based on the study design, therapeutic area and patient profile. Utilising innovative, world leading technology solutions and sites dedicated to accessing patients in specific therapeutic areas, provides our clients with comfort for on-time patient recruitment.
Some of the benefits these partners offer include:
- Streamlined EC (IRB equivalent) patient advertising and marketing approval submission processes
- Deep experience around EC advertising approval process – what is accepted
- Adherence to TGA (FDA equivalent) advertising/patient engagement regulations
- Complete understanding of Australian patient privacy and PHI regulations
- Rapid in-house or online screening
- Secure patient information transfers and connections with sites/Study Coordinators
- Feedback systems around recruitment strategies
Avance Clinical partners with three broad patient recruitment partner categories to ensure rapid patient recruitment for trials in Australia:
- Technology-based solutions
- Sites with specialist expertise and systems
- Patient services that support recruitment and retention
Technology-based solutions:
Digital technology solutions can offer speed and cost advantages as well as significantly de-risking patient recruitment. Avance Clinical leverages a number of world class technology solutions that support patient recruitment planning and rapid patient enrolment.
Pharma Intelligence | Informa
 Pharma Intelligence | Informa
Pharma Intelligence | InformaPharma Intelligence, Informa is a world leading market intelligence solution which provides precise and focused analysis for pharmaceutical, biotech, medtech, CRO, CDMO and investment and venture capital organizations. Avance Clinical utilizes Trialtrove and Sitetrove databases from Informa to accurately guide patient recruitment activities.
- Trialtrove is a comprehensive, accurate and up-to-date source of pharmaceutical clinical trials data and allows Avance Clinical access to patient recruitment benchmark data when planning for trials.
- Sitetrove provides comprehensive data on clinical trials sites and investigators and allows Avance to perform customized searches to:
- Generate lists of sites and investigators with the required experience and qualifications
- Use analytics to geographically identify investigator, physicians and patient data
Utilising this data from world leading databases such as Trialtrove and Sitetrove allows Avance Clinical to accurately plan for and drive rapid patient recruitment.
Following the study planning phase Avance Clinical has access to a number of technologically advanced solutions that facilitate fast patient recruitment including:
Evrima Technologies
 Charlotte Bradshaw, CEO and Founder, Evrima
Charlotte Bradshaw, CEO and Founder, Evrima Evrima Technologies
Evrima TechnologiesEvrima Technologies provides an automated, data driven approach to patient recruitment via precision matching, unlocking the 99% of potential trial participants not accessible through traditional recruitment methods. Evrima’s intelligent platform connects trials to physicians at scale and provides researchers with qualified participants for their trials via previously under-utilized electronic medical records.
Evrima’s CEO and Founder, Charlotte Bradshaw stated that “we have over 9 years of extensive patient recruitment experience across a wide range of therapeutic areas and have direct-to-clinician and direct-to-patient capabilities across Australia. Given that more than 80% of clinical trials are delayed due to patient recruitment challenges, Evrima’s core focus is to accelerate recruitment timelines and help Sponsor’s reach critical milestones rapidly.”
ClinTrial Refer
 Christine Zahren, Business Development Manager, ClinTrial Refer
Christine Zahren, Business Development Manager, ClinTrial Refer ClinTrial Refer
ClinTrial ReferClinTrial Refer is an award-winning Australian digital innovation developed by hematology clinical researchers in Sydney to solve the problem of patient referral and recruitment. Now widely used in other areas of medicine in Australia and overseas the mobile app and website platform allows doctors and patients to independently search for actively recruiting clinical trials and to access trial site locations and contact details in real time.
“ClinTrial Refer gives everyone access to clinical trials at their fingertips wherever they are – in the clinic, by the bedside or in team meetings. ClinTrial Refer can be licensed by any research group, hospital or network to increase the visibility of their clinical trials and improve recruitment” said Christine Zahren, Business Development Manager at ClinTrial Refer.
Contact Avance Clinical team for more information on how to access these world-leading and targeted technologies to help drive your patient recruitment efforts.
Sites with specialist expertise and systems – in alphabetical order
In addition to world-class recruitment technologies, Avance Clinical has excellent relationships with a very experienced and diverse array of clinical trials sites that drive patient recruitment across a broad range of therapeutic areas. These dedicated, private clinical trial sites are able to draw from distinct geographic areas and access patients from around the country. Many of these sites have established dedicated recruitment services to support their CRO and Sponsor clients.
 AusTrials Clinical Research
AusTrials Clinical Research Dr. Munro Neville, Managing Director, AusTrials
Dr. Munro Neville, Managing Director, AusTrialsAusTrials
AusTrials is a leading general medicine clinical research company with multiple sites in eastern Australia. Under a corporate agreement, AusTrials has access to the deidentified records from a nationwide network of primary care medical centres. These centres have world class facilities that support hundreds of independent General Practitioners (family doctors) and facilitate the recruitment to clinical trials. Managing Director and Study Investigator, Dr Munro Neville, stated that “AusTrials is currently enhancing a recruitment tool enabling superior matching of these 2 million patients to available clinical trials”.
 Paratus Clinical
Paratus Clinical Matt Clacy, Chief Commercial Officer, Paratus Clinical
Matt Clacy, Chief Commercial Officer, Paratus ClinicalParatus Clinical
Paratus holistically supports patient recruitment, starting with their dedicated Research Recruitment Specialists who understand the entire participant experience including the illness, intervention and schedule of events which assists in building strong participant relationships and reducing attrition. They work alongside experts to ensure every study has a bespoke plan created, which is dynamically managed by the Recruitment team who drive the recruitment process to help sponsors and CROs complete their studies quicker without any compromise on quality. Matt Clacy, Chief Commercial Officer at Paratus Clinical states that “Our clinics have ongoing relationships with referring partners and have developed captivating advertisements on an array of platforms to reach potential participants. We have also built an extensive database of participants with an array of conditions that are interested and keen to participate in clinical trials which ensures greater participant retention and trial efficacy”.
 Plexus Research
Plexus Research Suhit Anantula, Founder and CEO, Plexus Research
Suhit Anantula, Founder and CEO, Plexus ResearchPlexus Research – Adelaide, South Australia
Plexus Research is a community-based drug development partner with a rich and diverse Site Management Organisation network in Australia with a strong focus on recruiting patient populations through GP clinics. Through its network of clinics, combined with its data analytics platform, Plexus Research provides access to 100,000 active patients across a variety of demographics and disease groups. Their network of sites are community practices including both general and specialist practices. Founder & CEO, Suhit Anantula, states that “this provides access to a rich database of patients, and patient participation is increased due to the vicinity of sites to patients’ homes and continuity of care”.
 USC Clinical Trials
USC Clinical Trials Lucas Litewka, Director, USC Clinical Trials
Lucas Litewka, Director, USC Clinical TrialsUniversity of the Sunshine Coast (USC) Clinical Trials
USC Clinical Trials combines referrals from a network of specialists and general practitioners, close collaboration with patient advocacy groups and a database of more than 11,000 healthy volunteers to support its recruitment strategies. A key part of their recruitment strategy involves targeted social media advertisements and engagement with local media to rapidly reach patient populations. USC Clinical Trials tailor their engagement strategy to the community which has enabled recruitment numbers to exceed expectations across many therapeutic areas, with an average of 55 participants recruited per study over the last 2 years.
Lucas Litewka, Director, USC Clinical Trials states “Community engagement forms the backbone of our recruitment strategy. It’s our critical success factor in building trust and meeting rapid enrollment goals in healthy volunteer and chronic disease studies”.
Patient services that support recruitment and retention
Increasingly there is a diverse offering of unique services that engage with patients from screening through to study home visits. These services are also powerful patient retention partners.
 Affinity Clinical Research
Affinity Clinical Research Krys Hiscock, Managing Director, Affinity Clinical Research
Krys Hiscock, Managing Director, Affinity Clinical ResearchAffinity Clinical Research
Affinity Clinical Research is a patient-centric research organisation that specialises in providing support to research volunteers and investigators. Their team of home visit nurses leverage technology to move research into the community and into participant’s homes. The digital nature of the Affinity Clinical Research practice means that they are not limited by geography, with their local nurses providing essential support to ensure safety and retention of subjects. Krys Hiscock, Managing Director, advised that Affinity Clinical Research “conducts clinical trials across a wide range of therapeutic areas through partnerships with multiple medical specialists and doctors. This approach, along with an in-depth understanding of how patients navigate the healthcare system, increases the breadth and depth of our potential participant pool.”
 Illingworth Research Group
Illingworth Research GroupIllingworth Research Group (Australia) Pty Ltd
 Kevin Wightman, Senior Director, Business and Corporate Development, Illingworth Research Group
Kevin Wightman, Senior Director, Business and Corporate Development, Illingworth Research GroupIllingworth is the industry leader in provision of patient centric and site friendly GCP Research Nursing (RN) solutions. Illingworth’s mobile RNs provide patients and their carers with a flexible and practical way to participate in clinical trial visits from the familiarity and safety of their own home/workplace or school/college. All of their nurses are GCP trained and clinical trial experienced and are experts in providing personalized in-home trial visits and study procedures/assessments for patients who live remotely, are unable to travel, are immunocompromised or just leading busy lives. “This personalized and patient centric approach has been shown to increase study participation in terms of both initial recruitment and ongoing completion of clinical trial visits and can therefore dramatically impact data integrity, quality and trial completion timelines,” stated Kevin Wightman, Senior Director, Business and Corporate Development.
A combination of world class clinical trial intelligence databases, innovative recruitment technologies and a dedicated private site network positions Avance Clinical as the CRO of choice for supporting biotech’s with their early phase clinical trials.
Spotlight on Avance Clinical’s CRO Capabilities
Full-service CRO with a 20-year track record
eClinical
At Avance Clinical you have the option to deploy the latest eClinical solutions to maximise the value of your study data and deliver real-time visibility on study progress. Our team works with eClinical leaders such as Medrio to offer regulatory compliant and patient centric tools for rapid start-up and continual data flow. Improve patient engagement and data collection and stay informed at every step with Sponsor visible portals.
Some of the benefits of these advanced tools include:
Technology Partners:
Avance Clinical partners with best in class technology companies right across our service delivery to ensure a high quality, seamlessly integrated electronic solution for our clients. Our technology partners include the following:
- Medrio (eClinical Solitions)
- Medidata (eClinical Solitions)
- Trialogics (IWRS & ePro)
- MasterControl (eLearning Management System and eQuality Management System)
- Flex Databases (CTMS)
- Cetara – Phoenix WinNonlin (non-compartmental analysis (NCA), pharmacokinetic/pharmacodynamic (PK/PD)
In October, Avance Clinical accepted the MasterControl 2020 Innovation Excellence Award recognising our commitment to the use of world class technologies and innovation.
“Avance Clinical invests significantly in the industry’s leading clinical technologies making it one of the most advanced CROs globally for digital clinical research management and eClinical solutions,” said Avance Clinical’s CEO, Yvonne Lungershausen.
ClinicReady
 Gabriel Kremmidiotis
Gabriel KremmidiotisPhD, BSC Hon
Chief Scientific Officer
Avance Clinical’s ClinicReady team of scientific and medical affairs specialists comprise PhD qualified individuals with decades of experience in industry and academic research. They provide clients with scientific, regulatory and medical writing services, preparation of investigator brochures, clinical trial designs, study protocols, and patient information and consent forms as well as clinical trial data and clinical study reports.
“With our two decades of CRO experience we have become acutely aware of the importance of advising clients earlier in the development process, prior to them commencing preclinical safety and toxicology activities, so they conduct an appropriately balanced set of preclinical studies to get the right data required for approval of their first-in-human study in Australia,” said Dr Gabriel Kremmidiotis, Avance Clinical’s Chief Scientific Officer.
“Many of our clients are biotechs looking to get investor support to progress their project to demonstration of early Proof of Concept (POC) which will enable them to access return on their investment through a licensing deal to Pharma,” said Dr Gabriel Kremmidiotis.
With ClinicReady, Avance Clinical’s team can act as a surrogate drug development department for start-up companies.
Site Initiation Visit (SIV) and Study Start can be achieved in 5 – 6 Weeks.
There are two alternative schemes to gaining approval to conduct a clinical trial in Australia.
In Australia, the majority (>90%) of clinical trials can be submitted via the CTN scheme whereby preclinical data summarised in an Investigator Brochure are reviewed by an HREC who then notifies the TGA of the outcome. This provides an opportunity to conduct early phase trials in Australia whilst preparing documentation required for an IND submission to the FDA. Learn more here.
Watch our ClinicReady Informational Video here.