Boosting checkpoint inhibitor efficacy via the microbiome
Contributing authors: Peter Bak, PhD, Jonathan Barry, MD and Yechan Kang
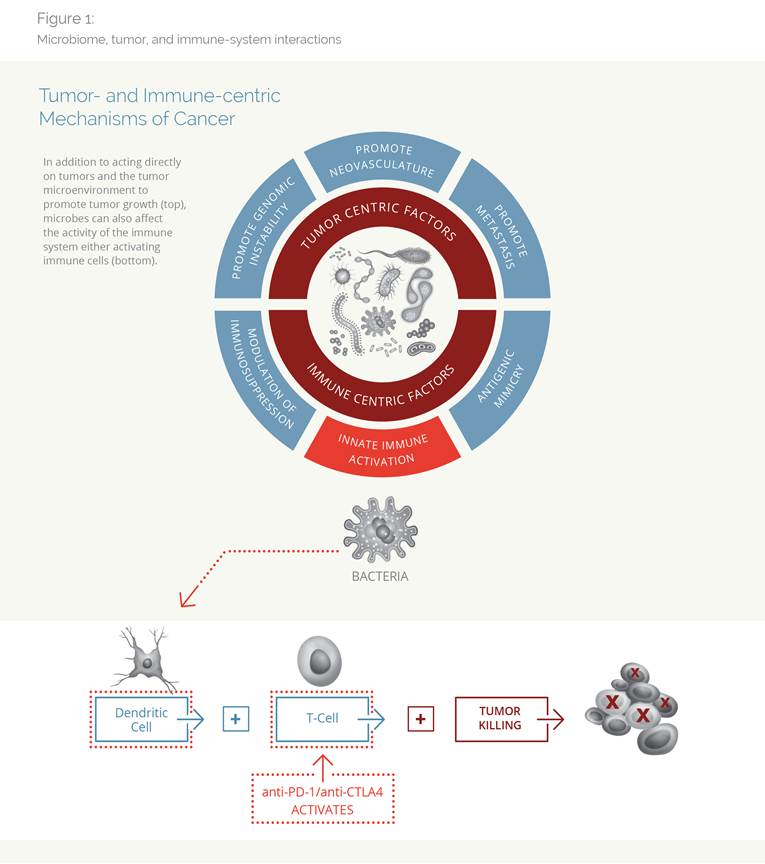
The latest generation of cancer treatments immunotherapies—a class of drugs called checkpoint inhibitors—has revolutionized cancer treatment. When they work, their results are astounding, but their effectiveness is an all-or-nothing proposition: not every cancer type is responsive to immunotherapy, and even in those tumors where immunotherapy radically changed the standard of care, only a fraction of patients respond to treatment. Faced with this tantalizing—yet frustrating—evidence physicians and researchers are searching for ways to deepen responses to immunotherapies and find a reason why some respond to therapy, and some don’t. Along the way, an unexpected factor has emerged—the millions of bacteria living within the human body.
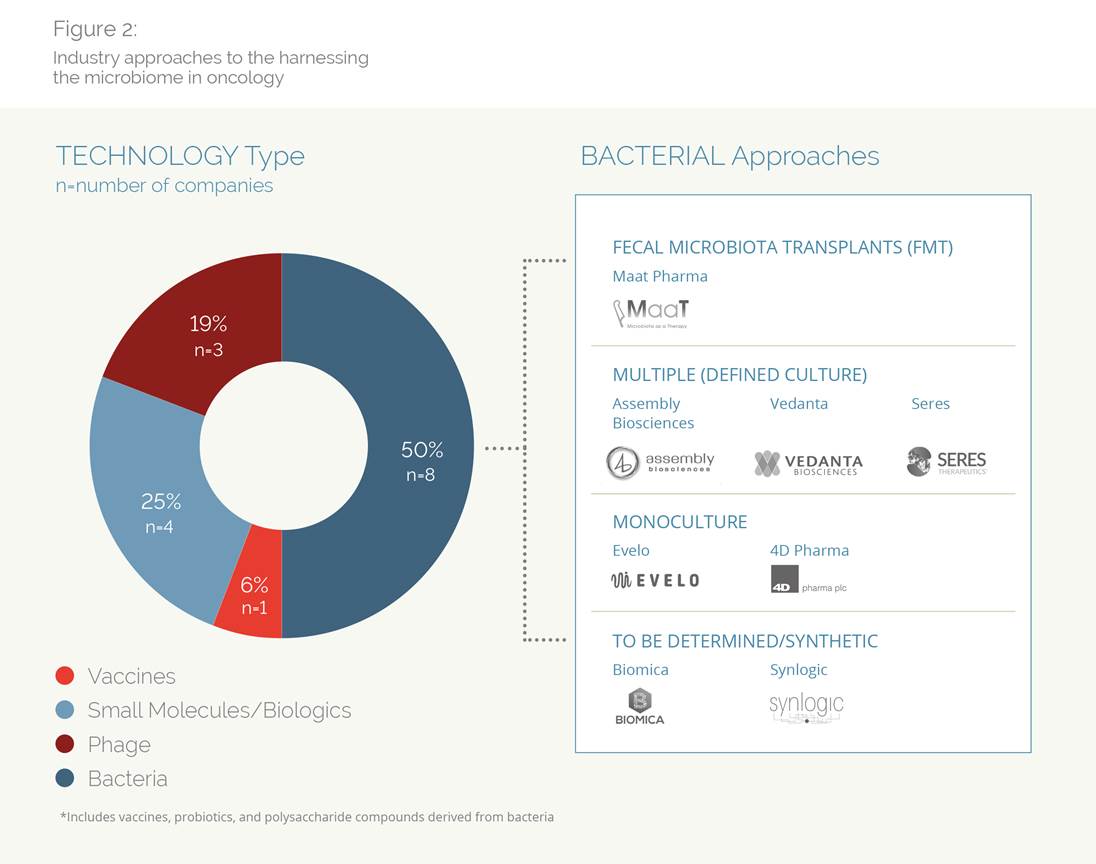
Several research teams, working with checkpoint inhibitors (CPIs) in mice, have found that fecal transplants from CPI-responsive animals conferred CPI efficacy to mice previously unresponsive to CPIs. The flurry of results in basic research has caught the eye of VCs, who have invested ~$500M in these companies over the past two years. As a mark that this science is entering “primetime,” some companies have also raised money from the public capital markets, most recently Evelo, who closed an $85 million IPO in May 2018.
Along with these advances in basic and early clinical science, the FDA has begun the process of sorting out when bacteria can be considered a “probiotic” versus a “drug,” and if the latter, how they might be considered within the established drug development path.
In 2016, the FDA took initial steps to pave a regulatory pathway for microbiome therapies with the publication of CMC (Chemistry, Manufacturing, and Control) guidelines for Live Biotherapeutic Products (LBPs). An LBP is defined as a product that 1) contains live organisms; 2) is applicable to the prevention, treatment, or cure of a disease or condition of human beings; and 3) is not a vaccine. This guidance was discussed at an FDA workshop held in September 2018, gathering clinicians, researchers, industry experts, and patient advocates to discuss key issues around clinical, manufacturing, and regulatory issues associated with microbiome therapies. In brief, microbiome therapies will be regulated as biologics, requiring an Investigational New Drug (IND) application and clinical trial data with standard clinical trials with standard outcomes for efficacy and safety, when the sponsor makes claims regarding the impact of a bacterial product on a disease state.
In addition to the regulatory considerations that must be taken into account when considering the commercialization of these technologies, the extent to which patents may be used to secure market exclusivity loom large. This topic is complex and multifaceted, with implications that span regulatory and investment considerations.
While many of the ongoing industry and academic-sponsored trials are looking to prospectively demonstrate an impact of FMT or bacterial cultures on patient outcomes, two key fundamental questions are top of mind for clinicians: why there is discordance between bacteria species and CPI responses? And, what is the definitive mechanistic link between changes in microflora and treatment response?
Regarding the former, each of the studies connecting CPI responders and non-responders to the presence of specific bacteria has identified a different genus and species of bacteria. As one physician put it: “What we have right now is a lot of association studies that do not agree with each other, nor make sense.” While multiple reasons may account for these differences, such as the way species were identified (e.g. differences in specific genetic evaluation, various stool collection techniques, and differences in geography, etc.), it begs the question of whether treatment will need to be specifically tailored to individual groups of patients based on race, geography, diet, etc.
While physicians realize the influence of bacteria may be multifactorial, and some for-profit development-stage companies may have generated proprietary data, this is seen as a stage-gating step for many physicians before they would recommend a microbe-based treatment.

With initial scientific reports as a backdrop, scientists, clinicians, and investors are racing to understand how the trillions of bacteria living within us be harnessed to fight cancer. There are still numerous questions leaving one wondering if the investment has outpaced the science:
Regardless, the potential return on investment is staggering. Current market leaders in the CPI space have recently posted upwards of $8 billion in sales within the current indication set.
Even in the most responsive tumors, a subset of patients respond; the majority of patients do not respond and discontinue treatment. Therefore, a microbial treatment that improves responses may be an extremely valuable asset in its own right and to the value to companies that currently market CPIs as a product that also increases the utilization of CPIs within established markets. Even more tantalizing is the opportunity to open up tumors where responses to immunotherapies have been historically poor. Tumors such as Glioblastoma multiforme, ovarian cancer, pancreatic cancer—some of the fastest-growing tumors—have not been responsive to single-agent CPI intervention.
With such clinical and economic potential, the next few years will be critical in assessing whether the fascinating early stage data will translate to meaningful patient outcomes, making ‘miraculous’ the standard of care.
Download the full report at https://bblsa.com/news-resources/whitepapers.