RWE challenges for today's biopharma
The rapid development of technology — and the resulting avalanche of data — are catalysts for significant change in the biopharmaceutical industry. This translates into urgent pressures for today’s biopharma, including a need to quickly and affordably develop products with proven therapeutic efficacy and value. This urgency is expedited by the growth of value-based contracting, where access to reimbursement and profit depends on these abilities.
Biopharmas cannot stay competitive by spending millions of dollars and several extra months to generate inflexible, siloed data that fails to deliver important insights of what is happening in the real world. In response to these changing demands, more companies are turning to real-world evidence (RWE) to provide enterprise value across both clinical and commercial use cases, while simultaneously driving increased time and cost efficiencies.
To explore the state of the industry and identify RWE use trends, SHYFT Analytics, a Medidata company, commissioned in-depth interviews with 20 RWE decision-makers across large, mid-size, and small biopharma companies.
Our research revealed a few key insights:
- All respondents believe that RWE use will continue to increase, and that their organizations are poised for more growth and development in the space.
- Except for a few large firms, most companies lack centralized decision-makers for RWE.
- Small and mid-size biopharma respondents worry about their ability to use a robust, end-to-end platform in an effective manner.
- Most prefer a “platform and services” model and a collaborative approach when working with RWE consultants and vendors.
- Most cited that RWE challenges are, in large part, RWD challenges, including processes and best practices for data identification, sourcing, validation, centralization, analysis, and application.
There is a massive and growing supply of RWD coming from the healthcare ecosystem today. While this influx of potentially valuable information offers opportunity, it also presents a major challenge for biopharma companies that lack the capabilities and resources to consistently meet industry fitness assessments for RWD and subsequent RWE.
While RWE is experiencing exceptional growth, companies still face many obstacles on their path to more efficient operations. Our study revealed that the majority of RWE challenges are deeply-rooted at an organizational and structural level. Apart from a small segment of large companies, most biopharmas lack the internal knowledge, expertise, and resources to effectively leverage RWE to improve business and research outcomes.
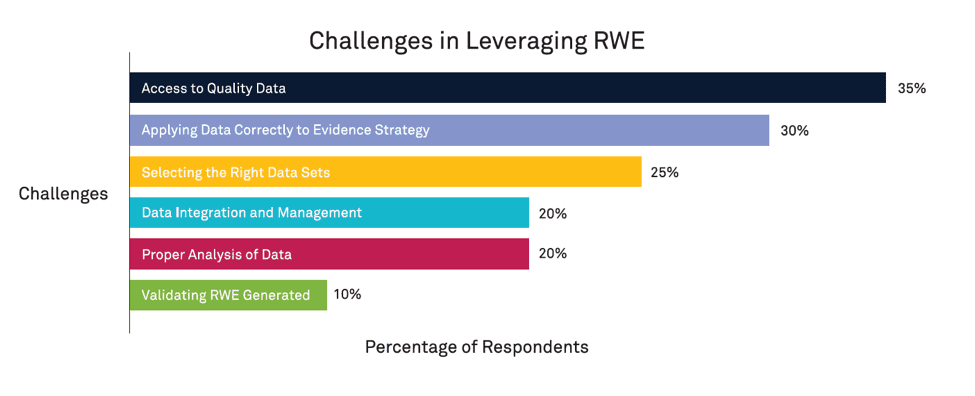
When asked about their organization’s top challenges in leveraging RWE, the majority of respondents indicated that RWE obstacles start with RWD. These challenges span the entire lifecycle of the data management process, from collecting and validating trustworthy data, to centralizing and integrating that data into their organization, to analyzing and applying it across specific use cases. Figure 1 shows the top-reported challenges among all respondents.
Top-reported challenges in leveraging RWE
35% of respondents cited difficulty accessing quality data due to barriers like data privacy laws and the speed of data acquisition. Another 30% reported challenges in determining how to use RWD to develop and implement evidence strategies that meet the needs of their products, business, or stakeholders.
This dilemma includes the selection of the data sets themselves — 25% of respondents felt ill-equipped to choose the right data from the overwhelming amount available.
Other important findings include issues with:
- Data management and integration, including standardizing, organizing, and ensuring new data is successfully integrated into existing data practices (20%)
- Lack of internal knowledge and expertise to robustly analyze data and generate meaningful insights (20 %)
The value of RWE is coming into focus and the potential is great, but many challenges remain. While different biopharma companies take different approaches to realizing the value of RWE, SHYFT’s research reveals a common theme across today’s industry:
“Regardless of whether they have a robust internal team or a complete reliance on partners, biopharmas are in search of a collaborative and dynamic “platform and services” RWE model — one that provides data collection, aggregation, and analytics capabilities that are only provided by an organization that specializes in RWE.”
This model helps to solve upstream RWD challenges such as collecting, validating, managing, and centralizing data, while ensuring that organizations receive guidance in data analysis, generation of meaningful RWE, and appropriate application and monitoring across use cases.
The market perspective report, The State of Real-World Evidence in Biopharma, contextualizes the findings from this research in an effort to provide a snapshot of where the industry is now, where it is heading, and how companies are allocating their resources to adapt and grow.