I-Mab —A Biotech on the Rise with Inspired Science
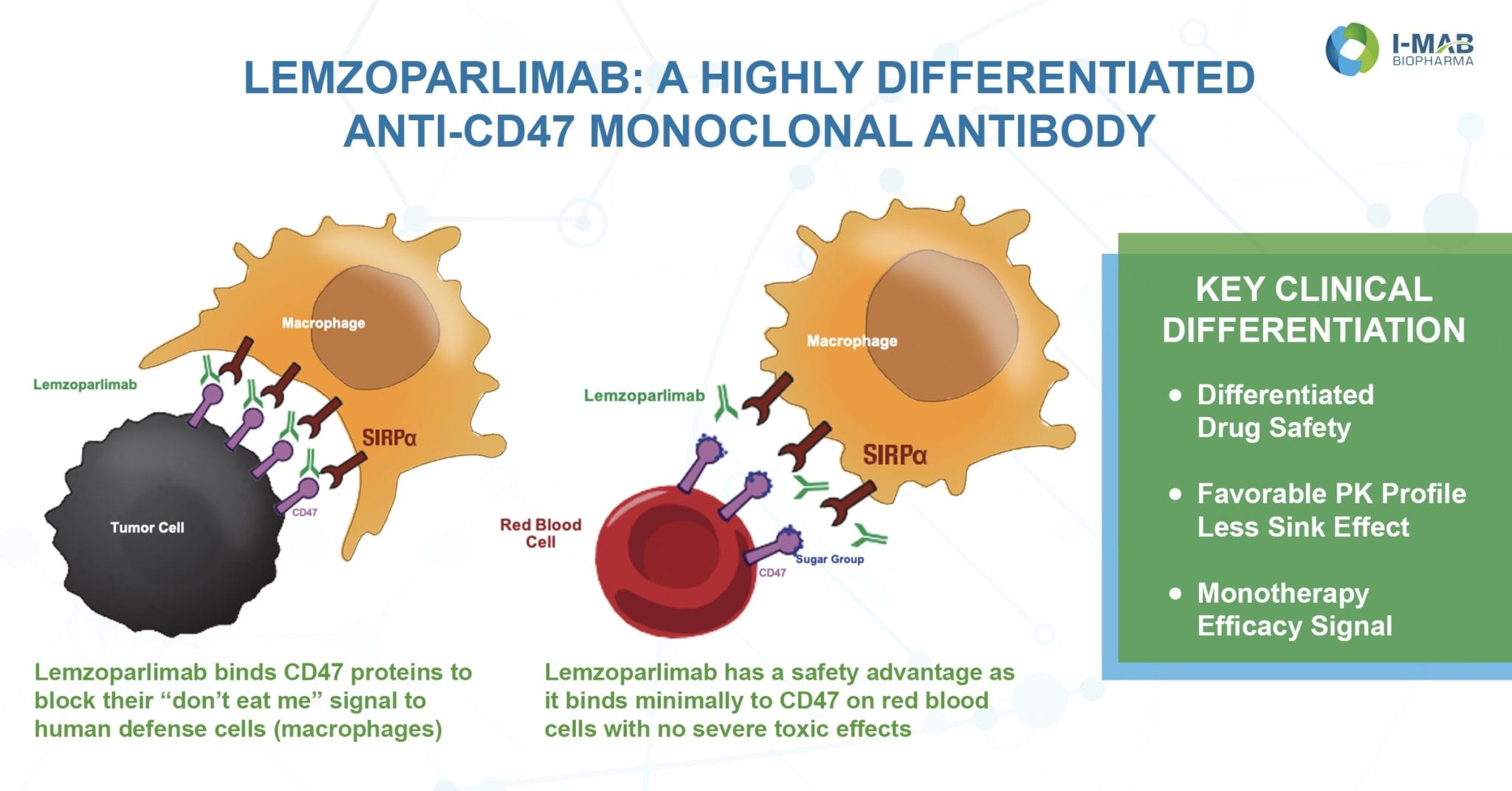
For Dr. Jerry Zhengyi Wang the journey to discovery started with a quest for a new way to leverage the immune system against cancer. His basic research on the role of macrophages in the anti-tumor immunity led him to CD47, which acts as a “don’t eat me” signal that enables cancer cells to evade detection.
Dr. Wang is among the I-Mab scientists behind lemzoparlimab, a highly differentiated anti-CD47 monoclonal antibody for the treatment of multiple cancers. The antibody is at the center of the global strategic partnership between I-Mab and the Chicago-based biopharma company AbbVie, announced last September and valued at about $3 billion. The potential of his team’s work to transform the treatment of cancer patients fueled an intensely focused investigation that began in 2016. And the entire process has been an incredibly fulfilling one, according to Dr. Wang, Vice President of Discovery.
“I have worked for multinationals, and you tend to just work at your own position; rarely do you have a chance to be involved in the entire drug development process,” said Wang. “Here at I-Mab you have a chance to discover something by yourself, and you can see how your own discovered drug develops and brings the benefits to the patients.”
Typically, CD47 is hindered as a cancer therapy by its side effect of severe anemia caused by natural RBC binding behavior.¹ I-Mab’s de novo discovery process took a unique approach to address this problem. Dr. Wang and his colleagues utilized a naïve human antibody library with around 10 billion diversified sequences and prioritized normal RBC binding assay in their screening to identify a unique clone with minimum RBC binding property and comparable tumor cell binding ability. Initial monotherapy results of US Phase I clinical trial data released in November demonstrated a differentiated safety and PK profile as well as efficacy signal for this antibody.
I-Mab is now expanding its US clinical trial of lemzoparlimab to parallel combination studies in solid tumors and non-Hodgkin lymphoma. In China, a separate clinical study in patients with AML/MDS is progressing well and has moved towards the highest dose (30mg/kg).
Click on the image to see the full-sized version
 Dr. Jingwu Zang, I-Mab Founder and Chairman
Dr. Jingwu Zang, I-Mab Founder and ChairmanDr. Wang is just one of many scientists who followed Dr. Jingwu Zang, I-Mab’s Founder and Chairman, to the company. Before founding I-Mab in 2016 and transforming it into a clinical stage company with a globally competitive pipeline in just four short years, Dr. Zang held posts as Senior Vice President and head of R&D China for GlaxoSmithKline, R&D head at Simcere Pharmaceuticals, and founding director of the Institute of Health Sciences and the Institute of Pasteur Shanghai. Both institutes are among the top research institutions in China. Dr. Wang trained in Dr. Zang’s lab in the Institute of Health Sciences and later joined him at I-Mab.
A significant factor behind I-Mab’s accelerated growth is the focus on inclusive leadership and cultivation of its people.
“I-Mab was founded with the idea that we could, and should, be truly innovative in building our differentiated pipeline,” said Dr. Zang. “In our pursuit of innovative drug programs, we attract and motivate a precious, growing talent pool of professionals with both global and local R&D expertise. This, coupled with critical mass of CRO services operating according to global standards, gives us remarkable power and speed in developing innovative medicines.”
I-Mab’s leadership team brings together decades of collective experience in drug discovery and development globally and in China markets. Left to right: CFO Jielun Zhu; Zheru Zhang, President and Head of CMC & Manufacturing; Founder and Chairman Jingwu Zang; CEO Joan Shen; and Chief Commercial Officer Ivan Yifei Zhu
Click on the image to see the full-sized version
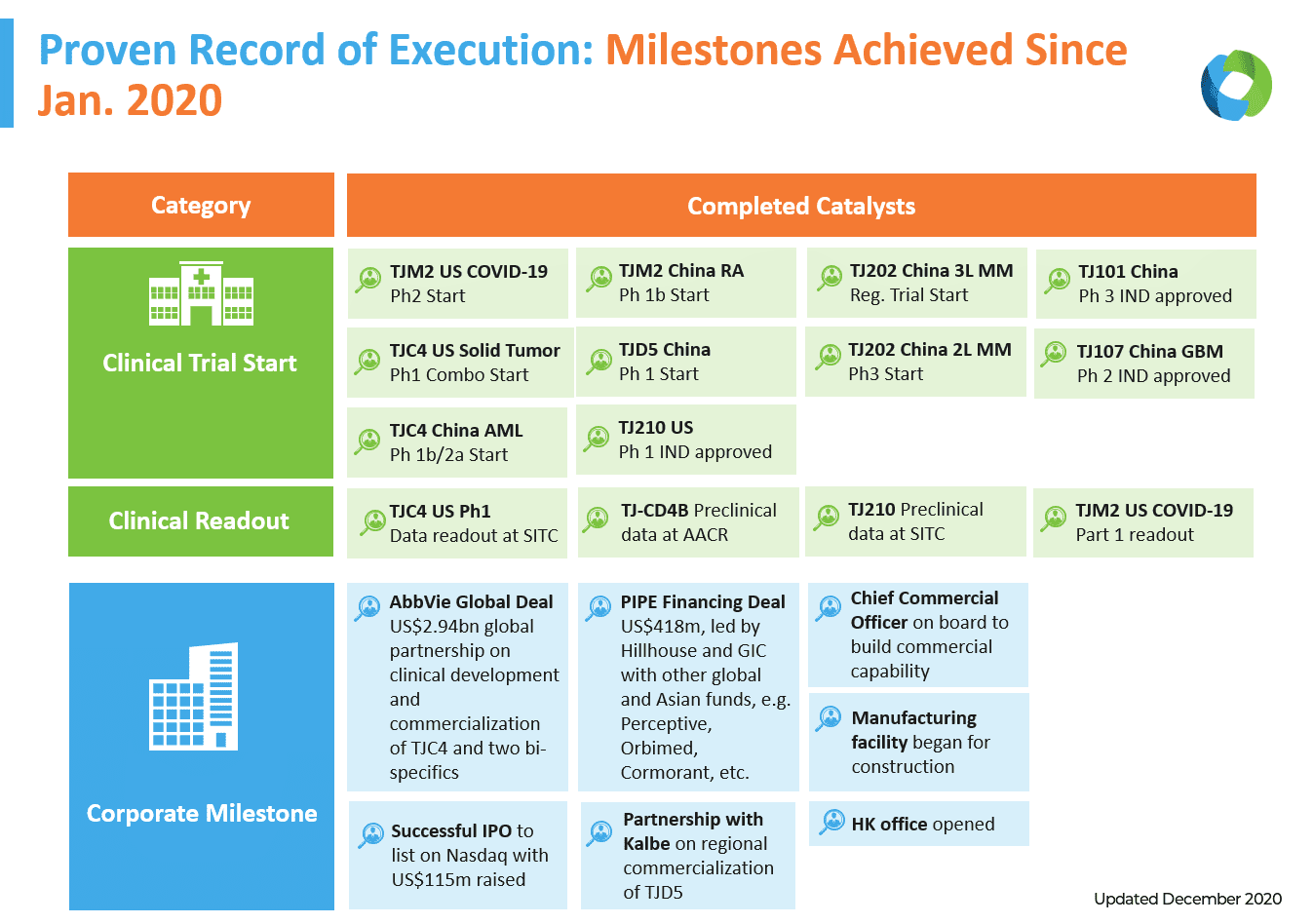
Indeed, long before the spotlight of CD47, I-Mab was already gaining industry attention and accolades for its unique business model—which combines a fast-to-proof of concept global approach in parallel with a fast-to-market China approach—to accelerate delivery on a pipeline of more than 15 assets in clinical and preclinical stages of development. Seven Phase I clinical trials are ongoing in the US and China, with another five Phase II and III trials in China and one Phase II/III trial in US. They include TJ107/HyLeukin-7™ for lymphopenic patients with newly-diagnosed glioblastoma multiforme (GBM) and TJ210, a novel antibody that blocks C5aR by binding to a unique epitope.
While it has been on a fast track since its establishment, I-Mab blew through several milestones in 2020, starting in January when it became the first China-based global biotech company to successfully complete a US IPO since 2017. Subsequently, I-Mab’s American Depository Shares (ADS) were selected for inclusion in the Nasdaq Biotechnology Index (Nasdaq: NBI).
In September, I-Mab completed one of the largest PIPE financing rounds in recent biotech history, raising approximately US$418 million through a private placement by a consortium of institutional investors led by Hillhouse Capital. This news came in parallel with the AbbVie deal.
“I-Mab’s progress as a company has been remarkable, especially over the last year,” said Michael Yi, Co-Chief Investment Officer of Hillhouse Capital. “This is a biotech to watch as it has shown that it can produce real innovation.”
Realizing its vision of becoming a fully integrated global biotech, I-Mab also began construction of its own state-of-the-art biologics manufacturing facility in Hangzhou, China. Establishing its own GMP manufacturing process is a strategic move by I-Mab to ensure quality, secure production slots, and maximize cost-effectiveness for clinical trial materials and commercial supplies, bolstering its ability to expand quickly in the years ahead.
With a focus on immuno-oncology, I-Mab has rapidly developed from its inception as a clinical stage biotech to a publicly listed company with more than 15 pipeline assets and 10 clinical programs.
Click on the image to see the full-sized version
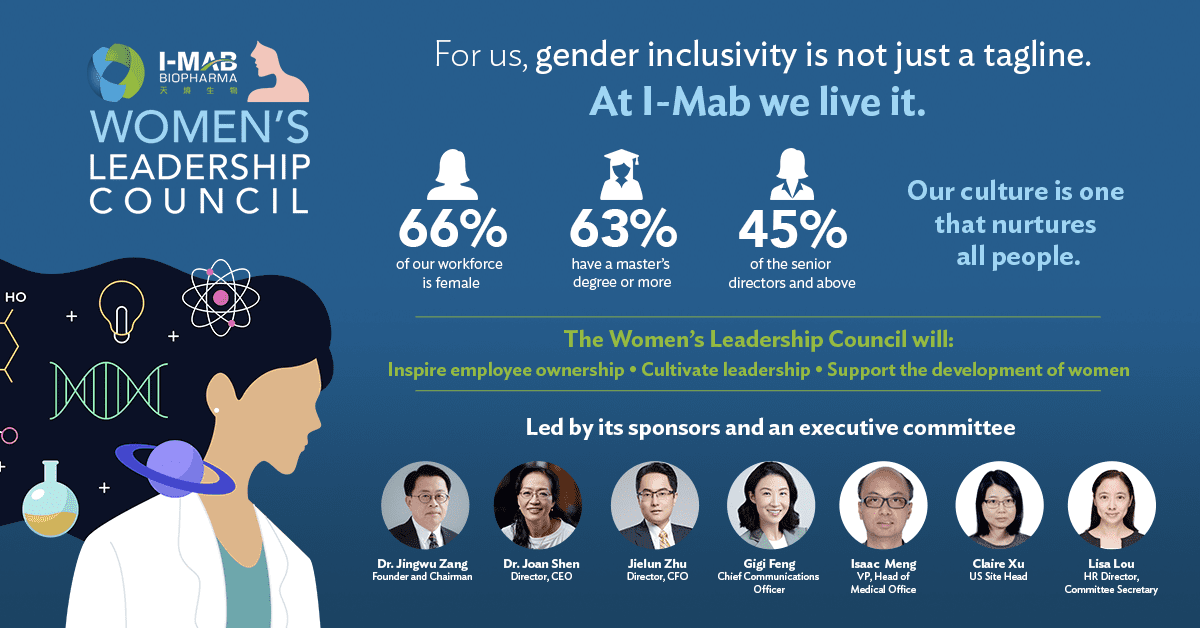
Another strategy for long-term growth is defining an inclusive culture. Amidst significant pipeline achievements and negotiation of partnerships, I-Mab also launched a Women’s Leadership Council (WLC) globally. Acknowledging the fact that pharmaceutical and biotech companies have the opportunity—and mandate—to create a healthier world by bringing more inclusive representation into the drug research and development process, the unique program aims to support the organization’s future female leaders to accelerate their career and personal development. This is being done through ambitious targets for more global leadership opportunities, exposure to the industry, and knowledge sharing across borders.
More than two-thirds of I-Mab’s employees are female, with 63% holding a master’s degree or above, and 45% of I-Mab’s senior directors and above are also female. The company has fostered an environment in which all people are given the opportunity to thrive.
Click on the image to see the full-sized version
“Our approach to our internal programs mirrors our approach to science in a sense. I-Mab’s culture strives to be mindful of the changing world around us as well as taking action on the urgent needs that we can address with our collective skillset,” said Dr. Joan Shen, CEO of I-Mab. “Looking into 2021, we look forward to more ambitious growth and cross border collaborations to accelerate innovation.”
¹ Huang Y, Ma Y, Gao P, Yao Z. Targeting CD47: the achievements and concerns of current studies on cancer immunotherapy. J Thorac Dis. 2017;9(2):E168-E174. doi:10.21037/jtd.2017.02.30