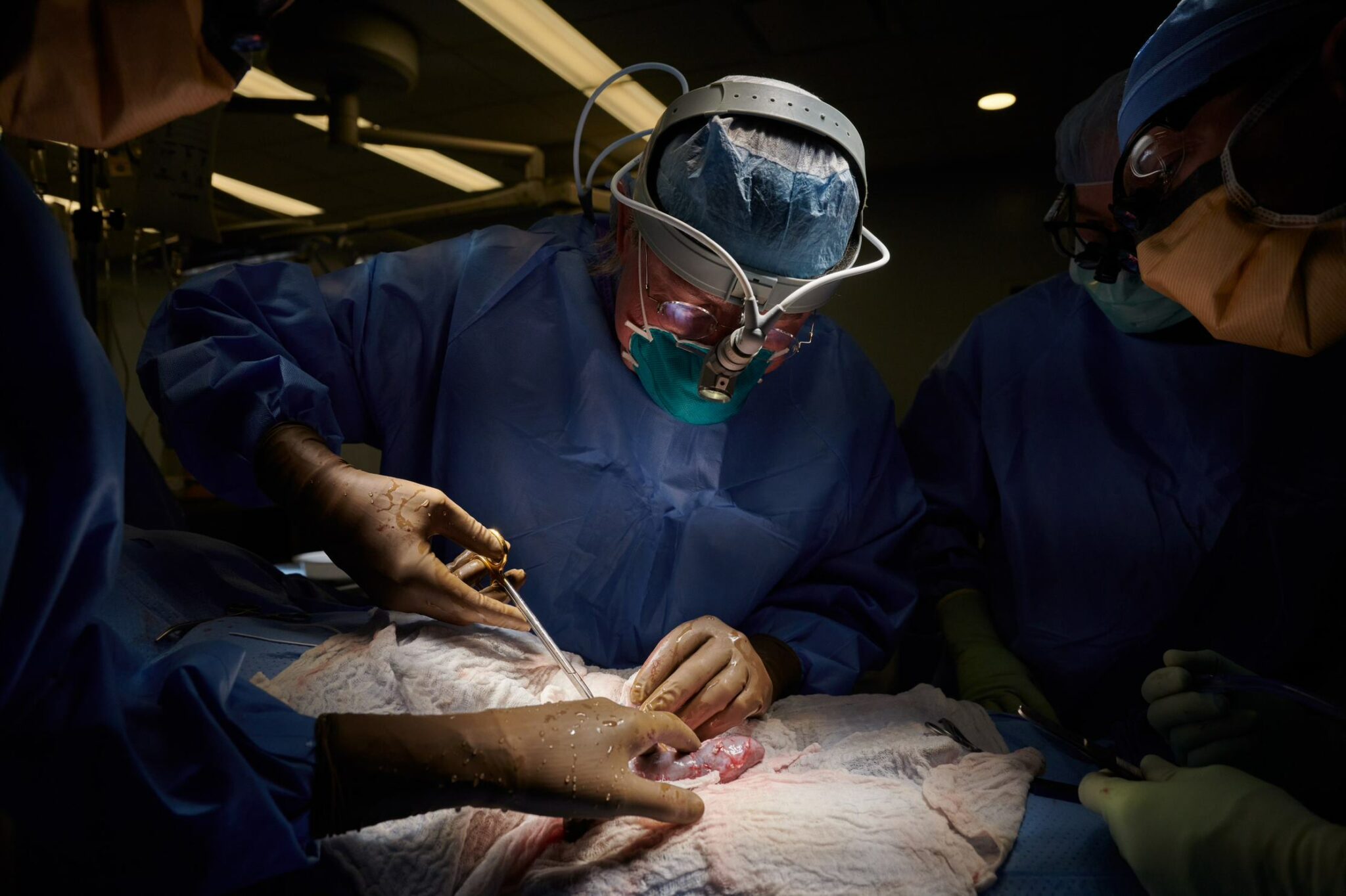
NYU surgeon transplants an engineered pig kidney into the outside of a brain-dead patient (Joe Carrotta/NYU Langone Health)
A virus may have driven the death of the first patient to receive a genetically modified pig heart
Doctors may be closing in on an answer for why the first human transplanted with a genetically modified pig heart died last month, around 60 …
Sign up to read this article for free.
Get free access to a limited number of articles, plus choose newsletters to get straight to your inbox.