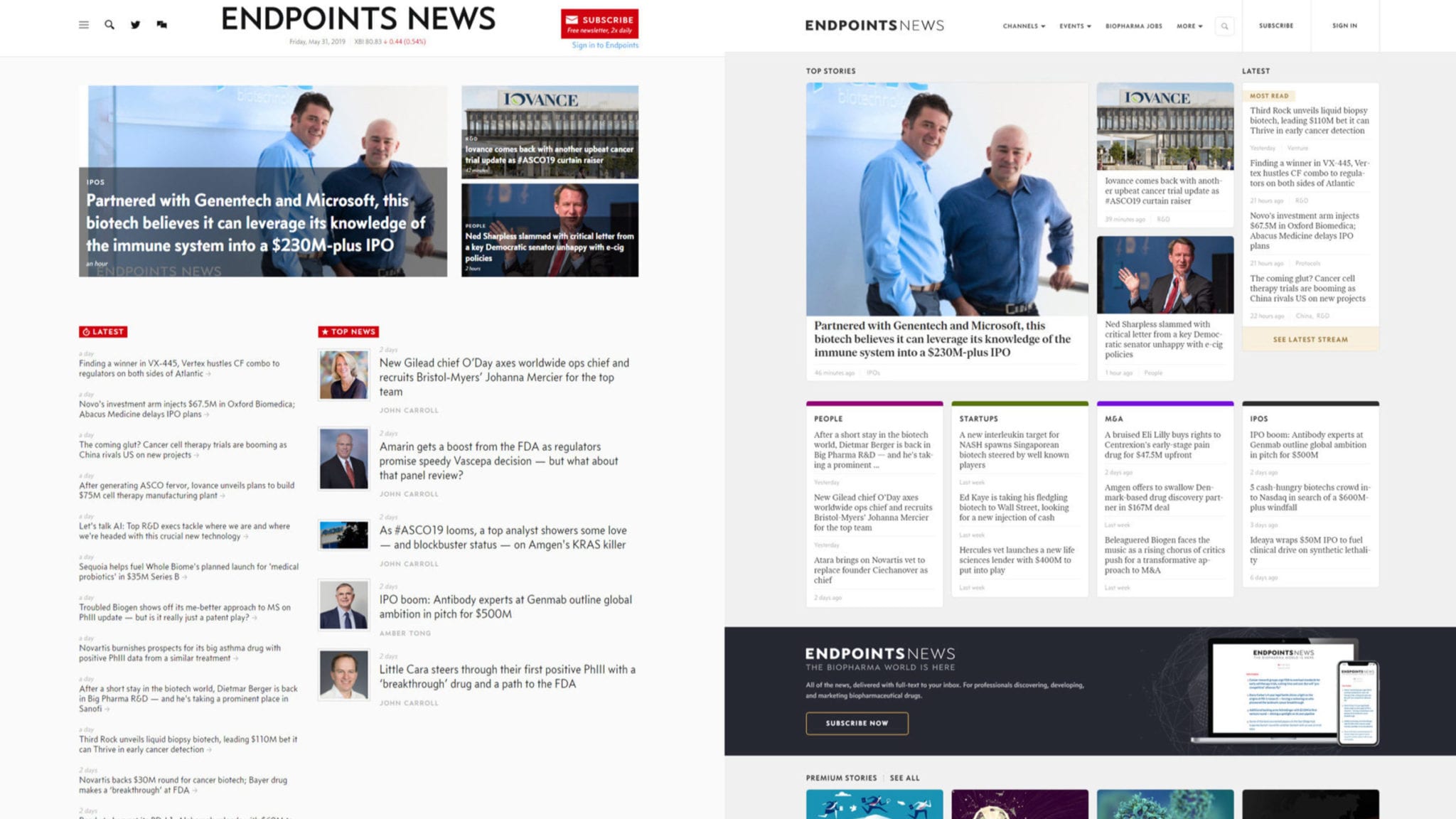
About the redesigned Endpoints News
We’ve upgraded the Endpoints web experience for the first time since our launch three years ago. It was built from scratch internally with the reader in mind to be a cleaner and more useable platform. We have some specific goals in mind for the design, which I’ll describe below. And for those interested, I’d like to go into some depth behind the overall process and backend behind Endpoints News.
Unlock this article instantly by becoming a free subscriber.
You’ll get access to free articles each month, plus you can customize what newsletters get delivered to your inbox each week, including breaking news.