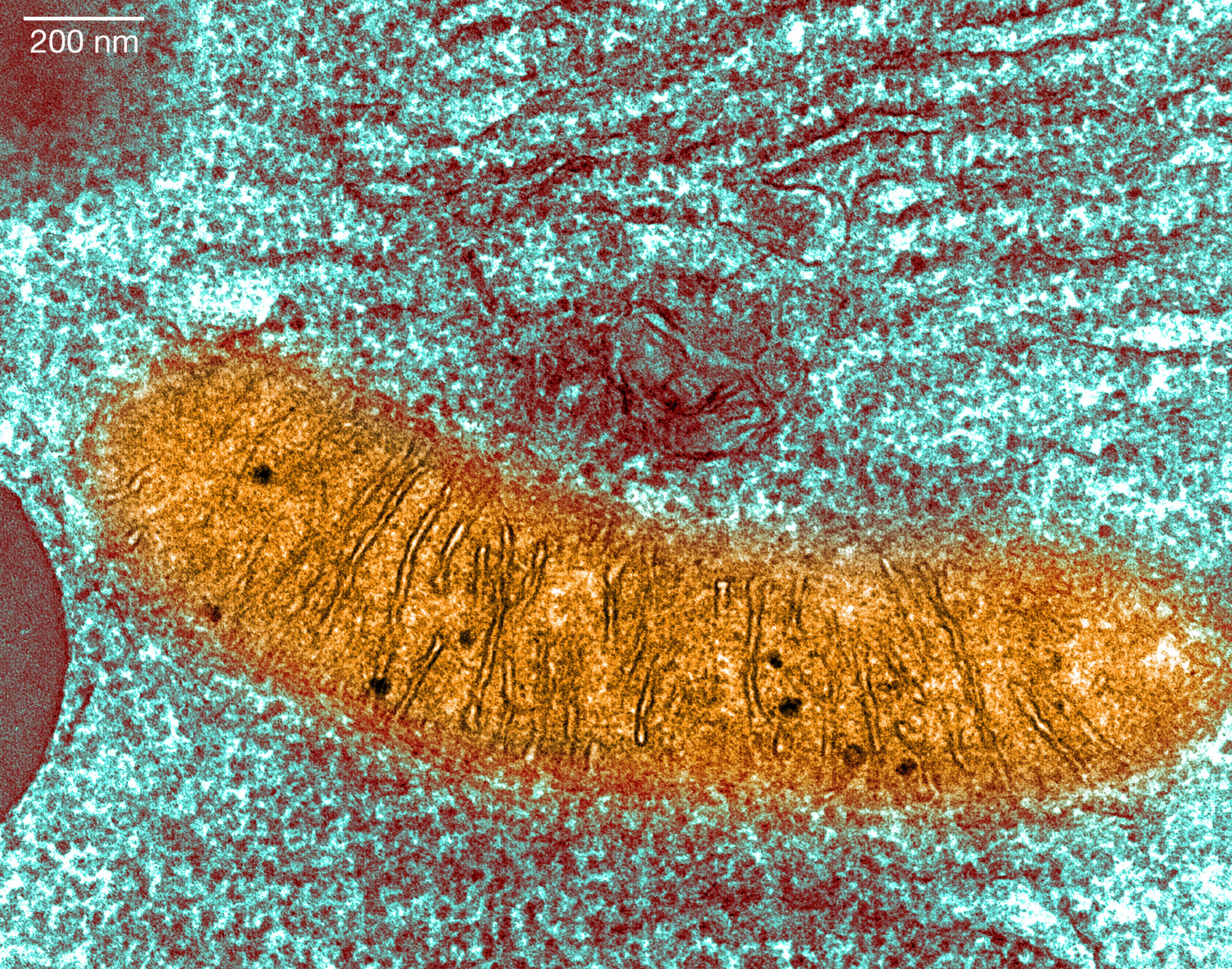
ECWYMB Transmission electron microscope showing mitochondria (Alamy Images)
Exclusive: ARCH and GV are building a large startup focused on mitochondrial diseases
ARCH Venture and GV, the venture arm of Alphabet, are building a large new company focused on mitochondrial diseases: rare genetic ones, initially, but eventually …
Sign up to read this article for free.
Get free access to a limited number of articles, plus choose newsletters to get straight to your inbox.