Photo: Julia Weeks/AP Images
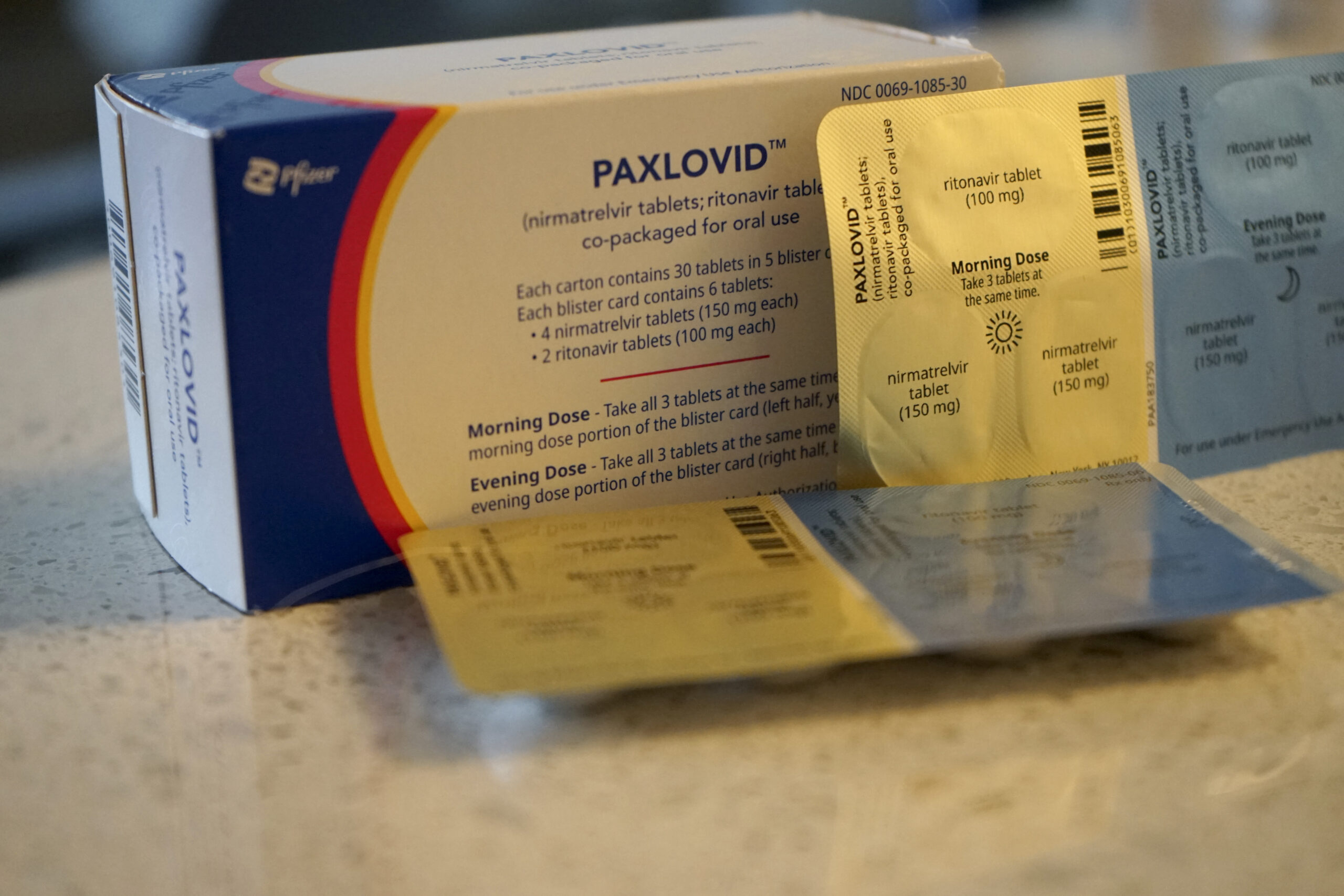
FDA axes requirement for positive Covid test before Paxlovid use
FDA announced today that doctors and pharmacists can now prescribe Paxlovid to patients without a positive test for Covid-19.
CDER Director Patrizia Cavazzoni
Sign up to read this article for free.
Get free access to a limited number of articles, plus choose newsletters to get straight to your inbox.