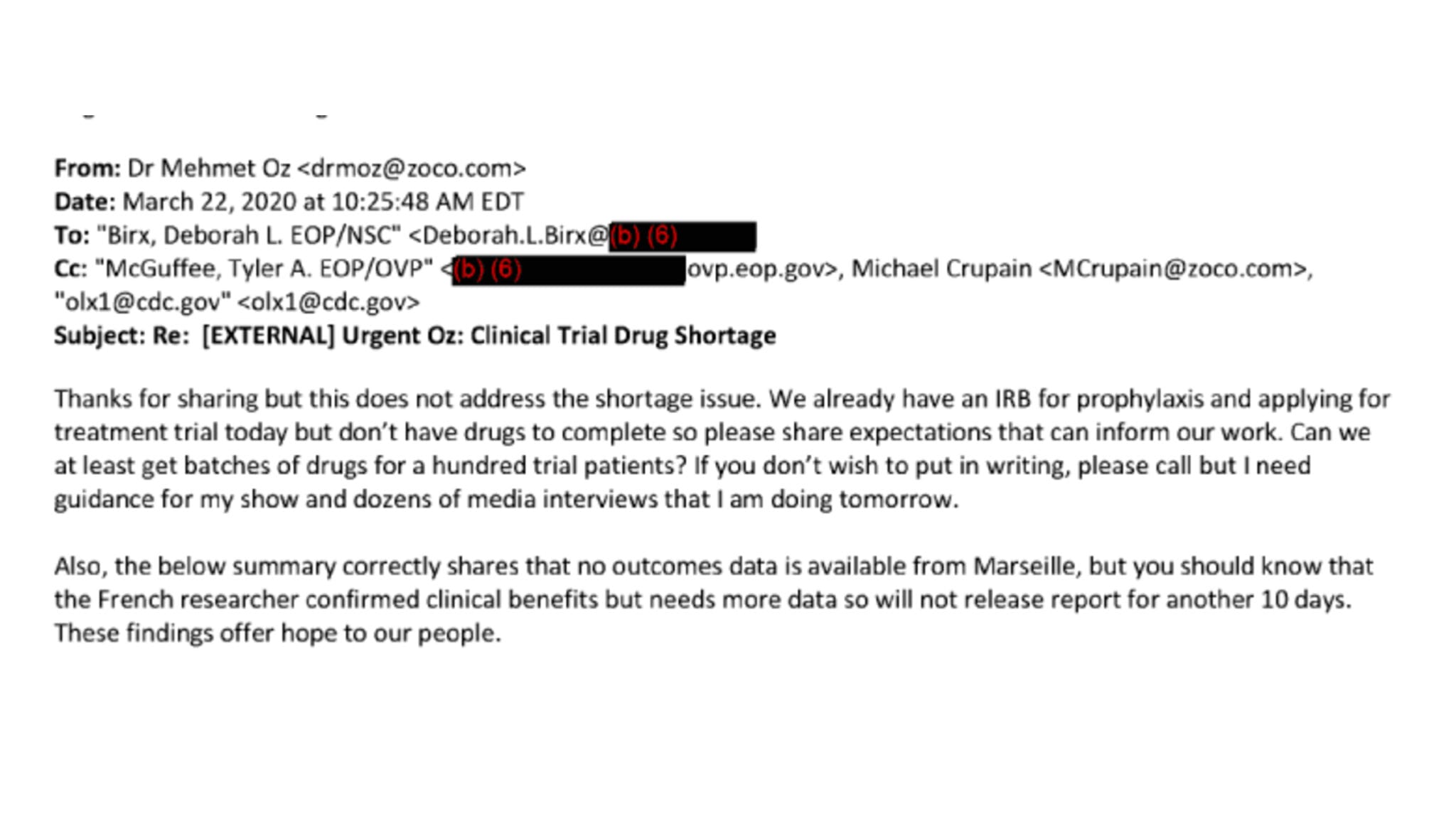
Internal Trump-era emails reveal a plethora of celebs, companies vying for FDA’s attention
The FDA on Thursday publicly released a trove of about 500 pages of internal and heavily redacted emails, showing how celebrities like Dr. Oz and …
Sign up to read this article for free.
Get free access to a limited number of articles, plus choose newsletters to get straight to your inbox.