Image: Shutterstock
Madrigal to grapple with lack of awareness about NASH ahead of potential FDA approval
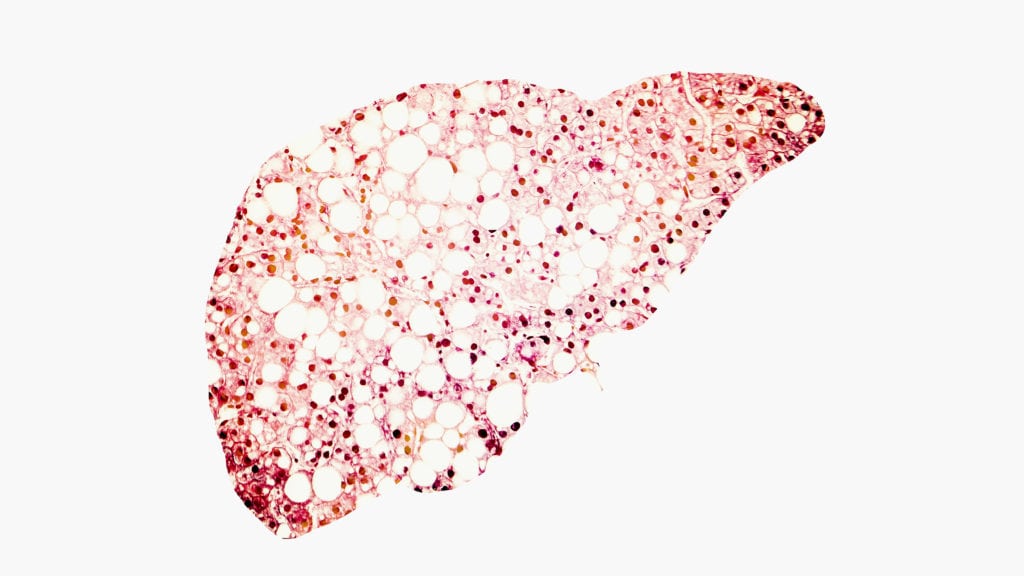
Madrigal Pharmaceuticals may be first in line to bring a NASH drug to market, but the company is also tasked with teaching Americans and their …
Sign up to read this article for free.
Get free access to a limited number of articles, plus choose newsletters to get straight to your inbox.