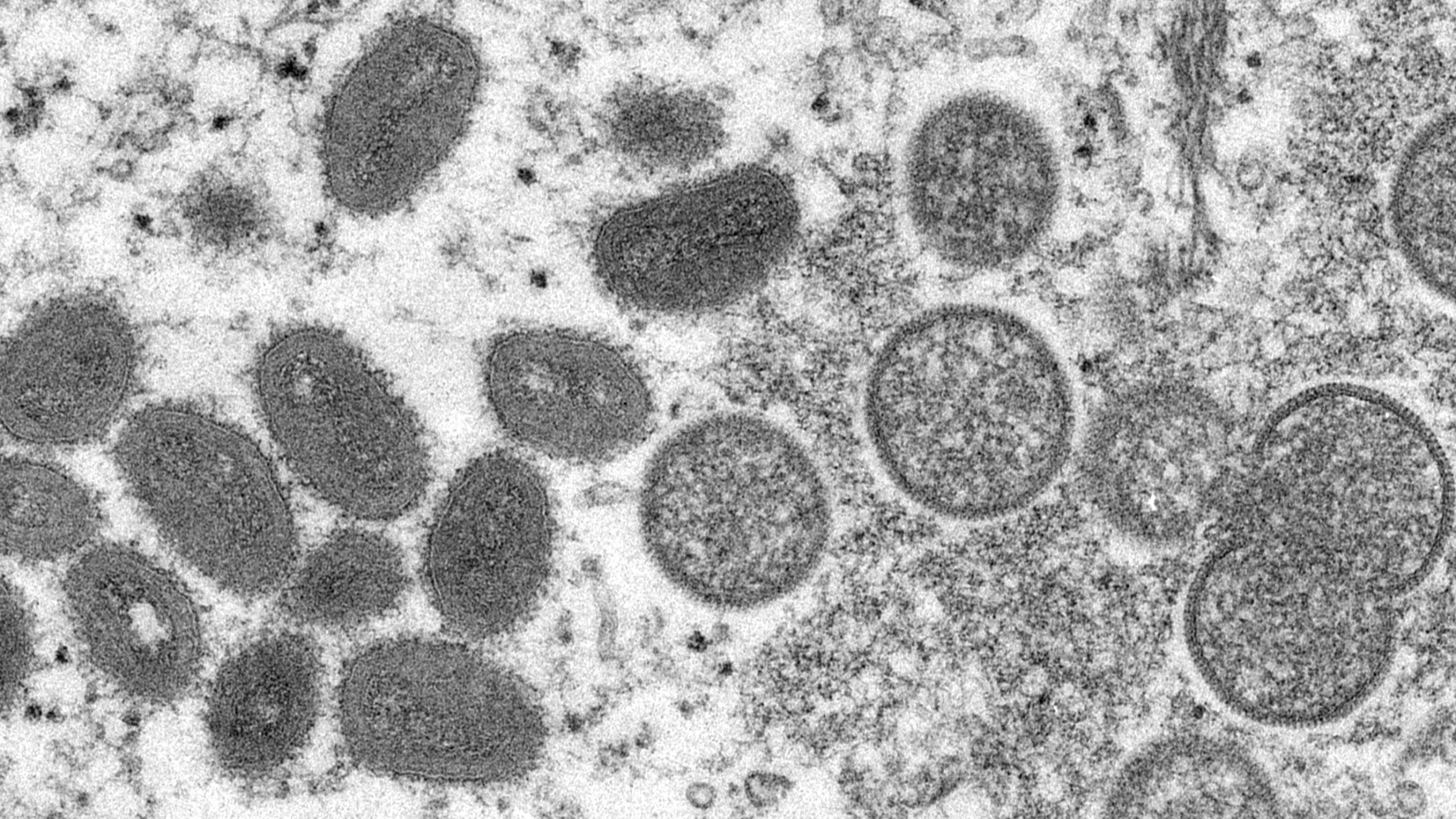
'Nothing escapes our scientists': A handful of biotechs gear up work on monkeypox
Two years after biotech and pharma sprung to action to create vaccines and therapies in record time for the rapidly circulating Covid-19 pandemic, a handful …
Sign up to read this article for free.
Get free access to a limited number of articles, plus choose newsletters to get straight to your inbox.