
2023 Spotlight on the Future of Drug Development for Small and Mid-Sized Biotechs
In the context of today’s global economic environment, there is an increasing need to work smarter, faster and leaner across all facets of the life sciences industry. This is particularly true for small and mid-sized biotech companies, many of which are facing declining valuations and competing for increasingly limited funding to propel their science forward. It is important to recognize that within this framework, many of these smaller companies already find themselves resource-challenged to design and manage clinical studies themselves because they don’t have large teams or in-house experts in navigating the various aspects of the drug development journey. This can be particularly challenging for the most complex and difficult to treat diseases where no previous pathway exists and patients are urgently awaiting breakthroughs.
Notably, this environment comes at a time when there’s an unprecedented amount of groundbreaking science coming out of smaller biotechs. In fact, of the nearly 7,000 drugs in active development by biotech today, a record 77% are from smaller companies.i

While today’s landscape presents some significant challenges, there is also much opportunity related to clinical development of these innovative medicines heading into 2023, that if realized, will bring immense value to the biotech industry – and society overall. For example, despite decades of effort and progress, there is still untapped opportunity to realize the impact that technology can have on the efficiency and quality of clinical research. For instance, modeling and simulation is just one avenue that offers significant potential to improve drug development. Furthermore, the current socio-political environment combined with the digital tools at our disposal today provides fertile ground to realize the potential of decentralized clinical trials – which can increase patient access and diversity, accelerate development, and generate stronger, more inclusive, and better representative data.
As Mark A. Goldberg, chairman and chief executive officer of Allucent explains, “In the face of the global, macro-economic challenges endured throughout 2022, it is important to focus on the persistent need for innovation and how we can help bring needed medicines to patients. There’s much reason to be hopeful based on the groundbreaking science coming from smaller biotech companies today and the forward-thinking drug development approaches we can leverage. That’s why we’re laser-focused on thinking big for small and mid-sized biotechs, and providing them with nimble, strategic solutions to help them succeed – and it’s why I’m extremely optimistic about what lies ahead for 2023 and beyond.”
Smaller Biotechs Have Experienced a Tumultuous Year, and Weathered it Well
Despite many upheavals affecting global business throughout 2022 – such the Covid- 19 pandemic and its aftermath, the war in Ukraine, and inflation – the outlook for small and mid-sized biotech companies remains strong. In fact, from a drug development standpoint, 6,918 clinical programs were reported in 2022 – up 6.3% versus 2021 – with 77% of them originating from small companies.i
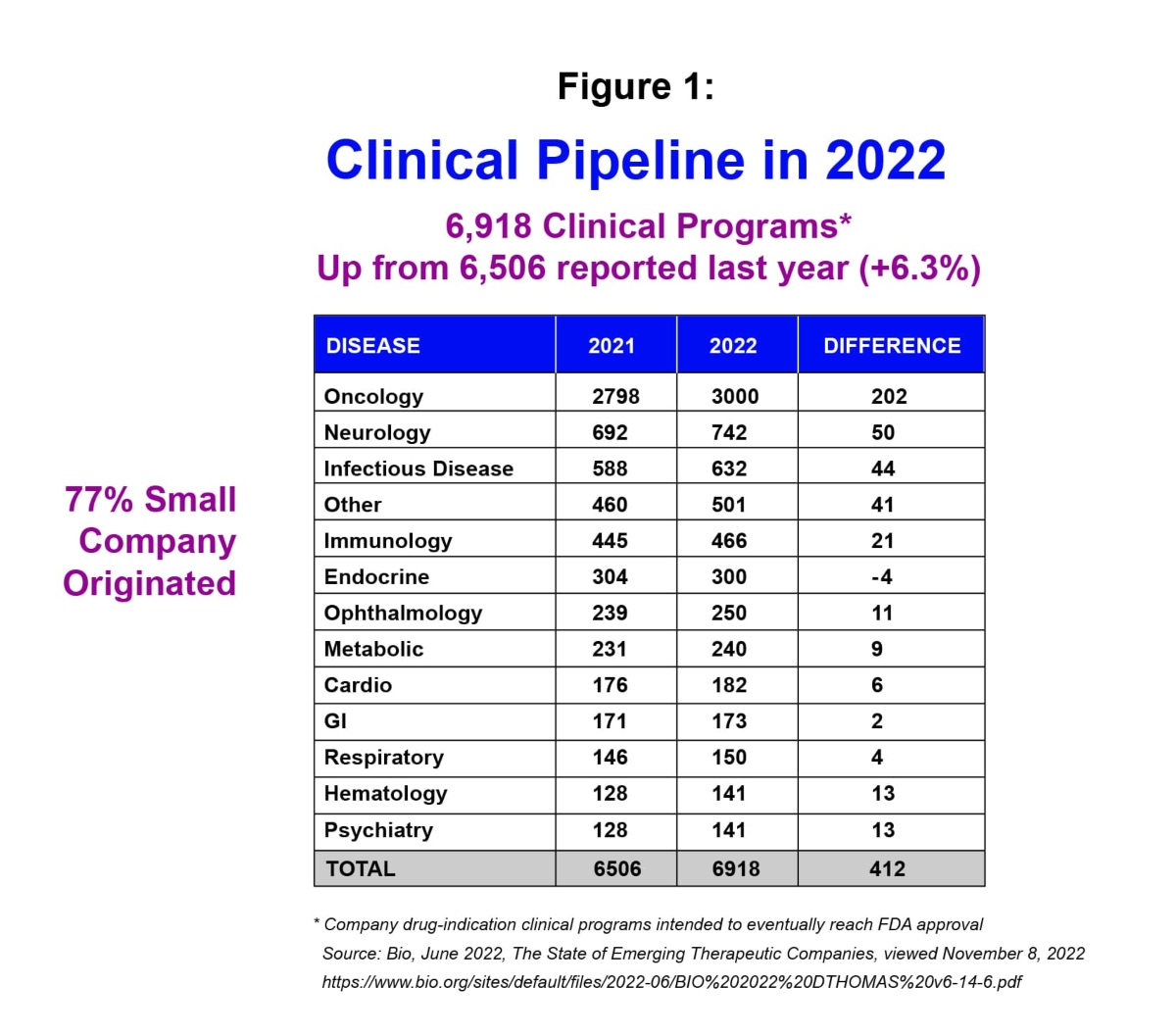
Moreover, in 2022 an estimated 70% of US FDA approvals for new treatments originated in small companies, compared to 66% in 2021.i
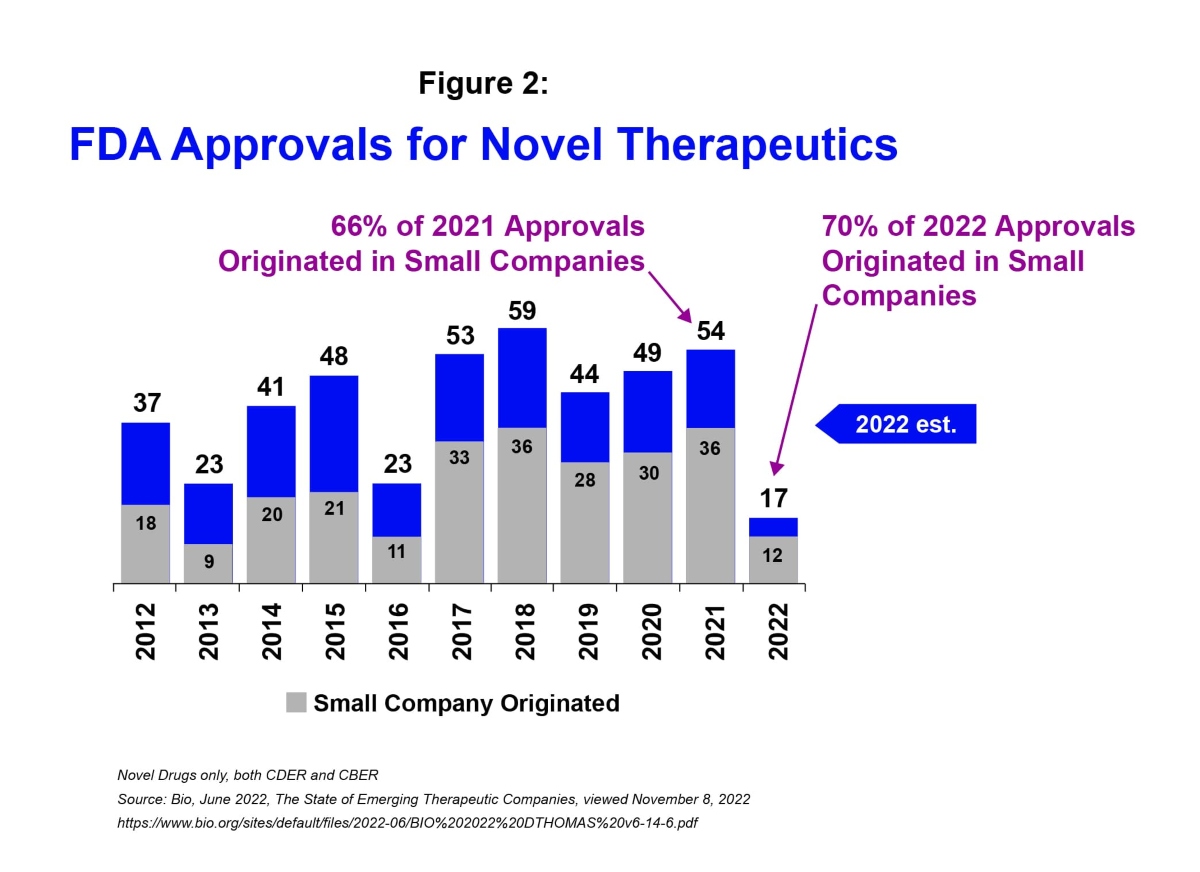
The scientific innovation coming from smaller biotechs and the number of clinical programs being put forth by these companies position the sector well heading into the new year. To realize the potential; however, a number of challenges will need to be addressed.
Improving Efficiency and Quality of Clinical Research Through Modeling & Simulation
The down-shift in investor sentiment that biotech is now experiencing began in late 2021, and is expected to continue for some time. As Barbara Ryan of Ernst & Young LLP explains in the EY 2022 Biotechnology Report, “We are clearly living through an innovation renaissance, and the fundamentals of the industry are quite strong. But from a stock market perspective, we are living through the deepest and longest correction that we’ve seen in the biotech indexes since their inception.”ii
Given this landscape, small and mid-sized biotech companies need to be particularly intentional about their decision-making process from the outset to help propel their science forward toward success. The inherent value in these companies is in the potential of their pipelines, which may consist of just one or two compounds for smaller-sized companies – making it imperative to make the right decisions and get their drug development programs off the ground on a strong footing and keep those programs moving forward efficiently and effectively.
One key lesson learned in the wake of the Covid-19 Pandemic is the need for the life sciences industry to employ more effective use of technology and data. From a drug development perspective, there are still many untapped opportunities to do just this. Today, techniques such as MIDD (Model Informed Drug Development) can be used to impact critical decision-making and, in some cases, reduce the need for certain trials.
MIDD integrates data and models from non-clinical and clinical programs, as well as data from other relevant external research, to increase the probability of success in developing medicines. It leverages a range of quantitative approaches to inform decision making, extrapolating data from large populations with similar characteristics to provide supporting evidence for safety, effectiveness, and dosing. These insights can be used to inform clinical trial design and predict trial outcomes – leading to more efficient, less costly, and more precise research. They can also help drug developers select appropriate doses for first-in-human (FIH) clinical trials and in special populations, such as renal and hepatic impairment or pediatric patients – a fundamental step in minimizing patient risk and increasing success rates.
A Ripe Environment to Improve Patient Access and Increase Diversity
Another key imperative heading into 2023 is the pressing need to improve patient access and ensure racial and ethnic diversity in clinical research. The greatest challenge in conducting clinical trials today remains recruiting and retaining patients – and the vast majority of patients who are recruited and retained are Caucasian.
Illustrating the disparities that exist currently, the US Food and Drug Administration’s Center for Drug Evaluation and Research released a report in 2020 indicating 75% of enrollees in clinical trials for novel therapies are white, with disproportionately low recruitment among non-white ethnic groups.iii
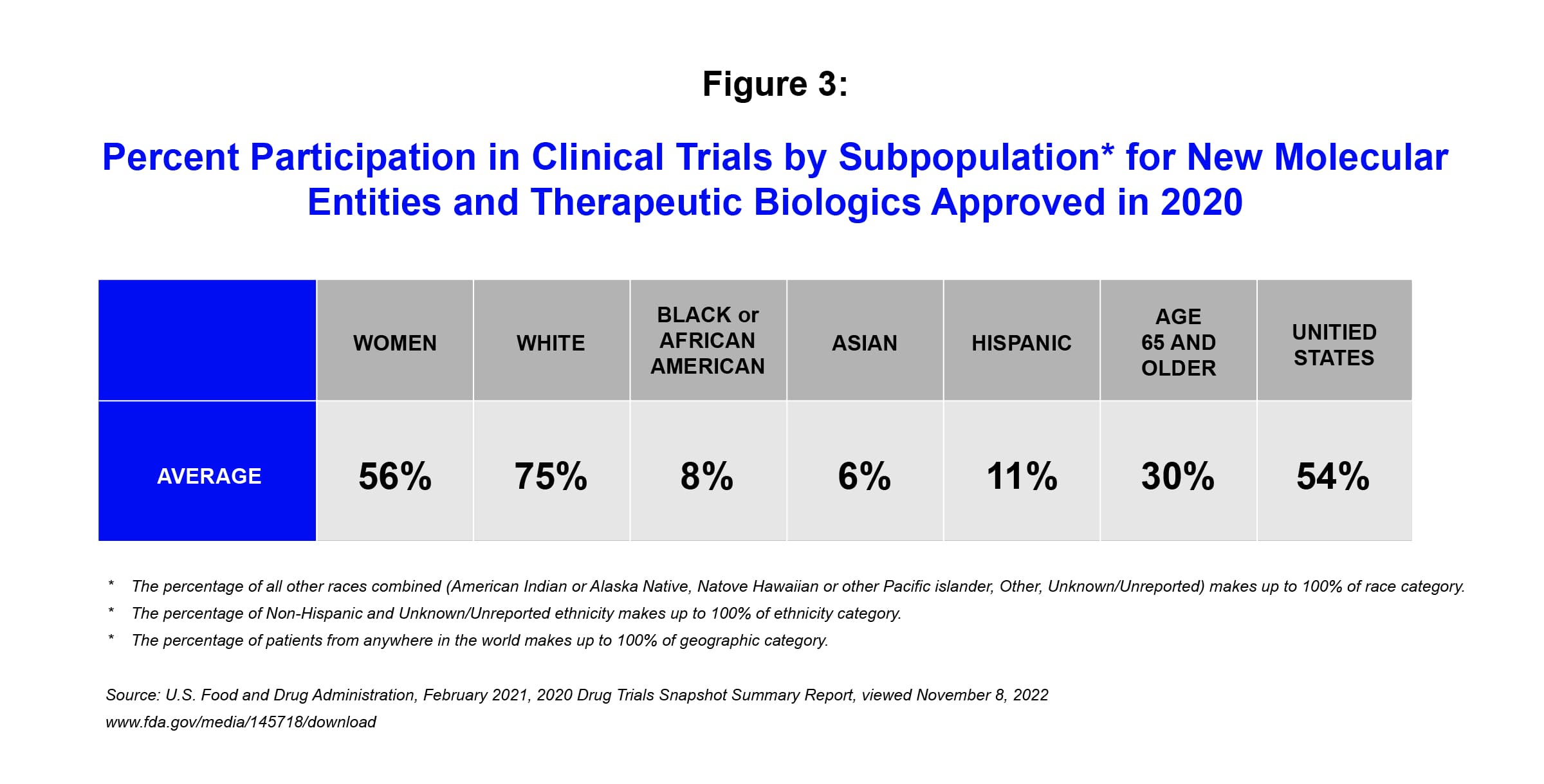
Race and ethnicity remain among the most significant social determinants of health today. Socio-economic factors play a significant role in affecting different health outcomes among different groups – but that is not the only contributing factor. It is well-known that there are important differences in how people respond to drugs based on their race, ethnicity and/or gender. To help ensure that medicines are proven both effective and safe for the people who need them, it is critical that the race and ethnicity of clinical trial populations be aligned with the epidemiology of the disease being studied.
Toward this end, in April 2022 the U.S. Food and Drug Administration released guidelines for sponsors developing medical products to create and submit a “Race and Ethnicity Diversity Plan” that outlines their plans for enrolling underrepresented racial and ethnic populations in clinical trials – providing the first-ever specific expectation by the FDA that sponsors develop diversity plans for their clinical research.
This is a highly-necessary and ethical endeavor. The challenge becomes that many small and mid-sized biotechs don’t have the resources of larger pharma companies to develop plans as outlined by the FDA, and may therefore benefit from external expertise and guidance.
Finding ways to decrease the burden on patients and lower barriers to participation is essential. Allucent is a strong-believer in the role that decentralized trial designs can play in achieving this goal. Decentralized and hybrid approaches can reduce or eliminate barriers to diversity and inclusion, enabling more representative patient access, and help accelerate clinical development and generate stronger data – all of which promise to benefit the industry as a whole and the patients we all serve.
Reasons for Optimism
The breakthrough science coming from small and mid-sized biotechs today has the potential to address many of the most complex and challenging diseases of our time. By putting in place the right experts, effectively employing the technology and data currently at our disposal, and keeping patients at the center throughout clinical development – small and mid-sized biotech companies are well-positioned to face today’s drug development challenges and deliver novel therapies to patients with unmet needs around the world.
References:
i Thomas, D. (2022, June). The State of Emerging Therapeutic Companies. BIO. https://www.bio.org/sites/default/files/2022-06/BIO%202022%20DTHOMAS%20v6-14-6.pdf
ii Ernst & Young. (2022). How do biotechs stay the course in uncharted waters? Beyond borders: EY biotechnology report 2022. https:///assets.ey.com/content/dam/ey-sites/ey-com/en_us/topics/life-sciences/ey-beyond-borders-2022-report-v11-web-hires.pdf
iii U.S. Food and Drug Administration Center for Drug Evaluation and Research. (2021, February). 2020 Drug Trials Snapshot Summary Report. https://www.fda.gov/media/145718/download