3 Tiers for AAV Potency Assay Development Success
Adeno-associated virus (AAV) gene therapies represent a major advancement in treating genetic disorders by delivering genetic material directly to patients’ cells. These therapies offer the potential for curative treatments by targeting specific genetic defects. However, their complexity necessitates rigorous testing to ensure efficacy. Among the various types of assays used, potency assays stand out as critical for understanding both the quality and strength of AAV therapies. These assays not only measure biological activity but also serve as indicators of stability, offering insight into the product’s long-term effectiveness. Due to their significance, potency assays are often a primary focus for regulatory agencies like the FDA and EMA, which require well-developed potency assays to approve AAV products. Forge Biologics supports developers across all clinical phases by offering a tiered approach to potency assay development, qualification, and validation, facilitating the progression of safe and effective therapies to market.
Regulatory Guidance on Potency Assay Design and Development is Evolving Requiring Adaptable Strategies
As AAV gene therapies advance through clinical trials and reach the market, regulatory guidance on potency assay requirements continues to evolve. The FDA’s 2011 Guidance for Industry and the International Council for Harmonisation’s (ICH) Q6B guidelines provide comprehensive frameworks for potency assays, with recent updates emphasizing assay qualification and validation throughout the product lifecycle1. While early-phase clinical trials may allow more flexibility, requirements become increasingly stringent as products advance towards commercialization.
In late 2023, the FDA released a draft guidance titled, “Potency Assurance for Cellular and Gene Therapy Products,” which further clarifies expectations for potency assays2. This guidance highlights the need for potency assays to measure biological activity and ensure product consistency and stability, elevating their role in the overall quality control strategy for gene therapy products.
Complexities of AAV Potency Assay Development
Developing potency assays for AAV therapies requires thoughtful approaches to navigate common challenges. Early-phase assays focus on proof-of-concept, often using in vitro reporter assays involving transfection with plasmids encoding a gene of interest (GOI). These assays are quick and flexible, allowing adjustments as understanding of the product’s mechanism of action (MOA) evolves.
As programs move into Phase I/II clinical trials, the focus shifts to developing quantitative assays that reliably measure biological activity and begin to define potency criteria. This process requires time and creativity due to variability in starting materials like novel vectors, transgenes, and cell types, with vector design–serotype, promoter, GOI, etc.—all focused on ensuring efficient, targeted, and safe in vivo delivery. However, AAV transduction mechanisms remain unclear, and in vitro cultures lack the cellular diversity and extracellular support found in vivo, leading to differences in tropism and transduction efficiency3.
A functional in vitro potency assay depends on selecting a cell line that expresses vector-derived RNA under the chosen promoter, and, if applicable, translates it into a functional protein. Once a suitable cell line is identified, transduction conditions are optimized for the vector serotype. Since AAV therapies often target intricate biological pathways, understanding or defining the MOA may be difficult. Developing, qualifying, and validating functional potency assays that reflect the MOA is essential as programs progress towards commercialization.
Forge Biologics’ Tiered Approach to Potency Assay Development and Qualification
Forge Biologics offers a tiered approach to developing quantitative potency assays, providing flexible support to developers at various stages of therapeutic development. This approach evaluates potency through three tiers: gene expression, protein expression, and functional potency. Here, we apply Forge’s tiered potency development approach to a hypothetical gene therapy using an AAV vector to upregulate a deficient protein’s production (Fig. 1).
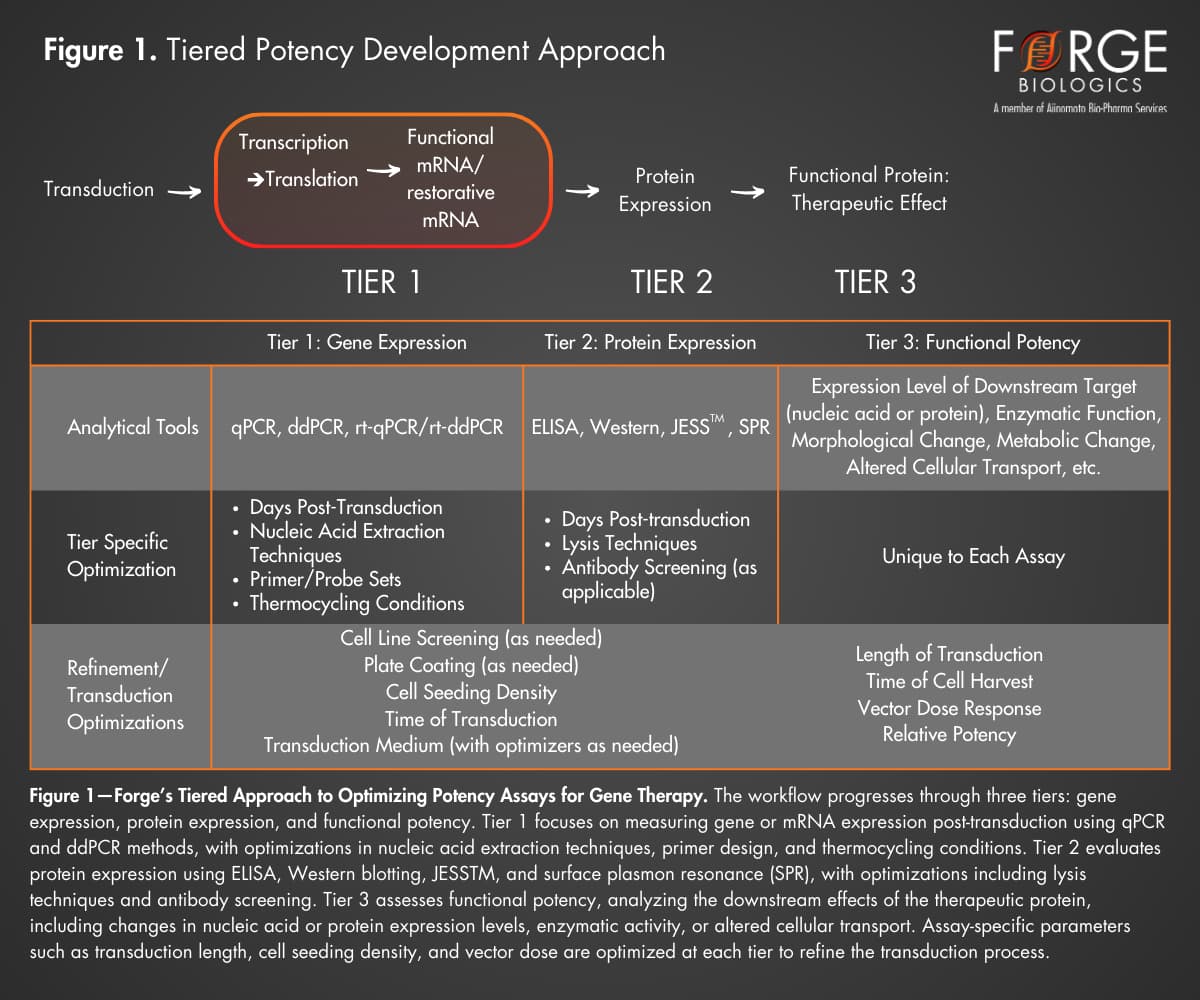
- Tier 1: Quantification of Gene Transfer. This initial tier focuses on quantifying gene transfer and expression of the gene of interest (GOI). Transduction studies are performed in HEK293 cells or client-specific cell lines, optimizing conditions like cell seeding density, multiplicity of infection (MOI), plate coatings, and serum replenishment. RNA is isolated and converted to cDNA and nucleic acids are quantified in-house using techniques like quantitative PCR (qPCR) and Droplet Digital PCR (ddPCR) to measure gene transfer efficiency.
- Tier 2: Quantification of Protein Expression. The second tier assesses whether the transferred gene is translated into the intended protein, and whether the protein is expressed at measurable levels. Transduced cells are lysed to extract proteins, which are then quantified using ELISA, Western Blot (including automated systems like JESSTM), or similar techniques. This stage provides insights into the potential therapeutic efficacy and offers a stability-indicating measurement for potency.
- Tier 3: Functional Potency. In the final tier, functional potency assays evaluate the biological activity of the expressed proteins in a therapeutically relevant manner. Since measurement of functional activity is intricately linked to the intended therapeutic outcome, functional potency assays can be highly unique from product to product. Forge’s Analytical Development (AD) team collaborates closely with clients to design and qualify functional assays that reflect the MOA of the product. Techniques such as flow cytometry, High-Performance Liquid Chromatography (HPLC), ELISA / SPR, and enzymatic activity assays can be used to evaluate functional activity.
Qualification of Potency Assays
Following development, potency assays are refined and qualified by Forge’s Analytical Development (AD) and Quality Control (QC) teams for clinical lot release testing. Qualified assays demonstrate accuracy, precision, specificity, range of quantitation, and robustness, ensuring lot-to-lot consistency and supporting data-driven release specifications.
Forge’s Innovative Strategy for Superior AAV Transduction
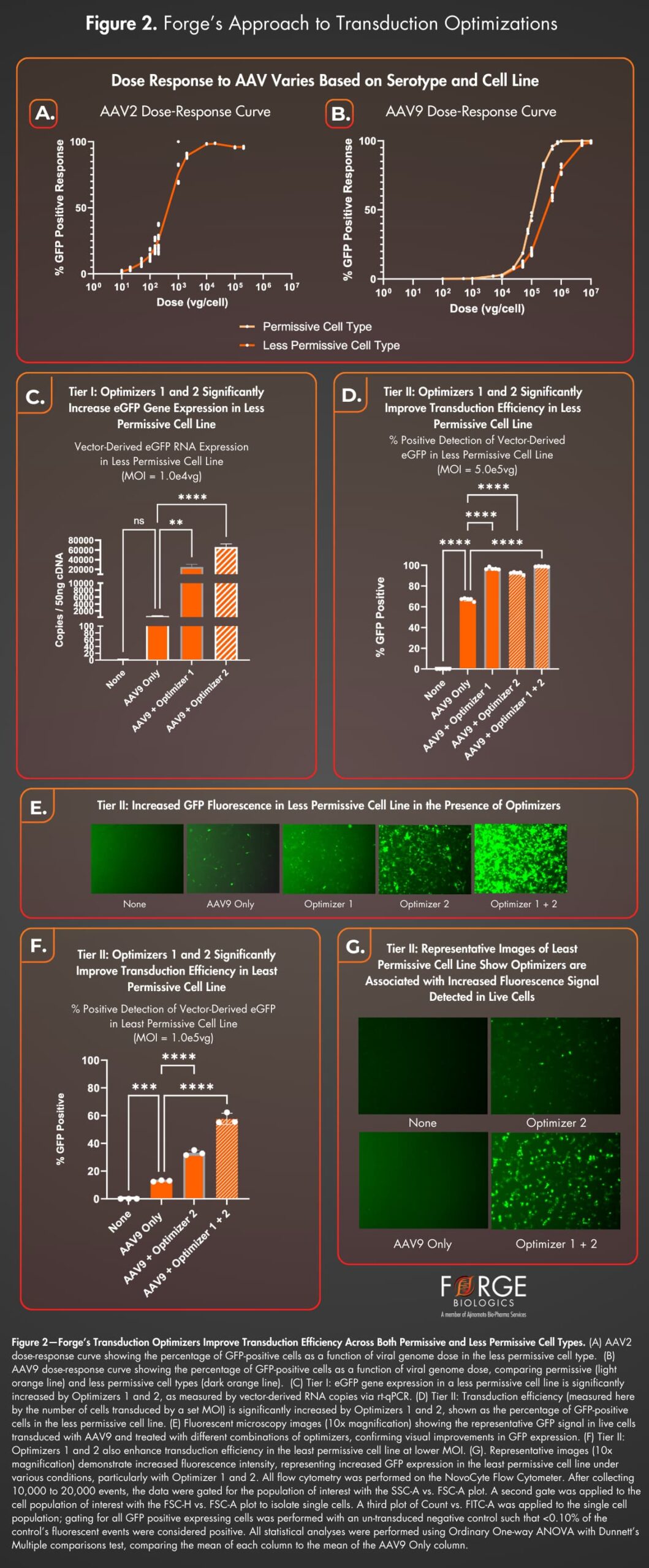
Optimizing transduction efficiency is crucial for AAV gene therapy potency assays due to the variability in infectivity across different serotypes and cell lines. Forge has developed a method to improve transduction efficiency through dose-response experiments and innovative use of transduction enhancers.
As part of Forge’s potency optimization strategy, dose-response curves were generated for AAV9.eGFP vectors in both a “permissive” cell line and “less permissive” cell line, and for AAV2.eGFP in the “less permissive” cell line using flow cytometry (Fig. 2A, B). Our data highlights the contrasting infectivity profiles of AAV2 (highly infectious in vitro), and AAV9 (less infectious but clinically relevant), emphasizing the need for transduction optimization. These dose response curves lay the groundwork for transitioning to relative potency estimation using parallel-line and parallel-logistic assays, which are becoming the industry standard.
Forge has pioneered a transduction optimization strategy for AAV9 in both cell lines using two transduction enhancers, “Optimizer 1” and “Optimizer 2.” Combining these enhancers—a novel approach within the industry—resulted in a synergistic transduction increase (Fig. 2C-F).
Tier I improvements in the “less permissive” cell line were measured by eGFP gene expression (Fig. 2C), while Tier II included advanced flow cytometry analysis (Fig. 2D). Live-cell imaging showed a significant increase in expression with combined optimizers, not captured by flow cytometry alone (Fig. 2E). In a third “least permissive” cell line, Optimizers 1 and 2 again yielded significant transduction improvements (Fig. 2F, G). Optimizer 1 was not tested independently in this experiment but showed smaller effects in a separate study (data not shown). These findings showcase the effectiveness of these optimizers in enhancing transduction in challenging cell types.
Effective Technology Transfer of Potency Assays from Developer to a CDMO Partner
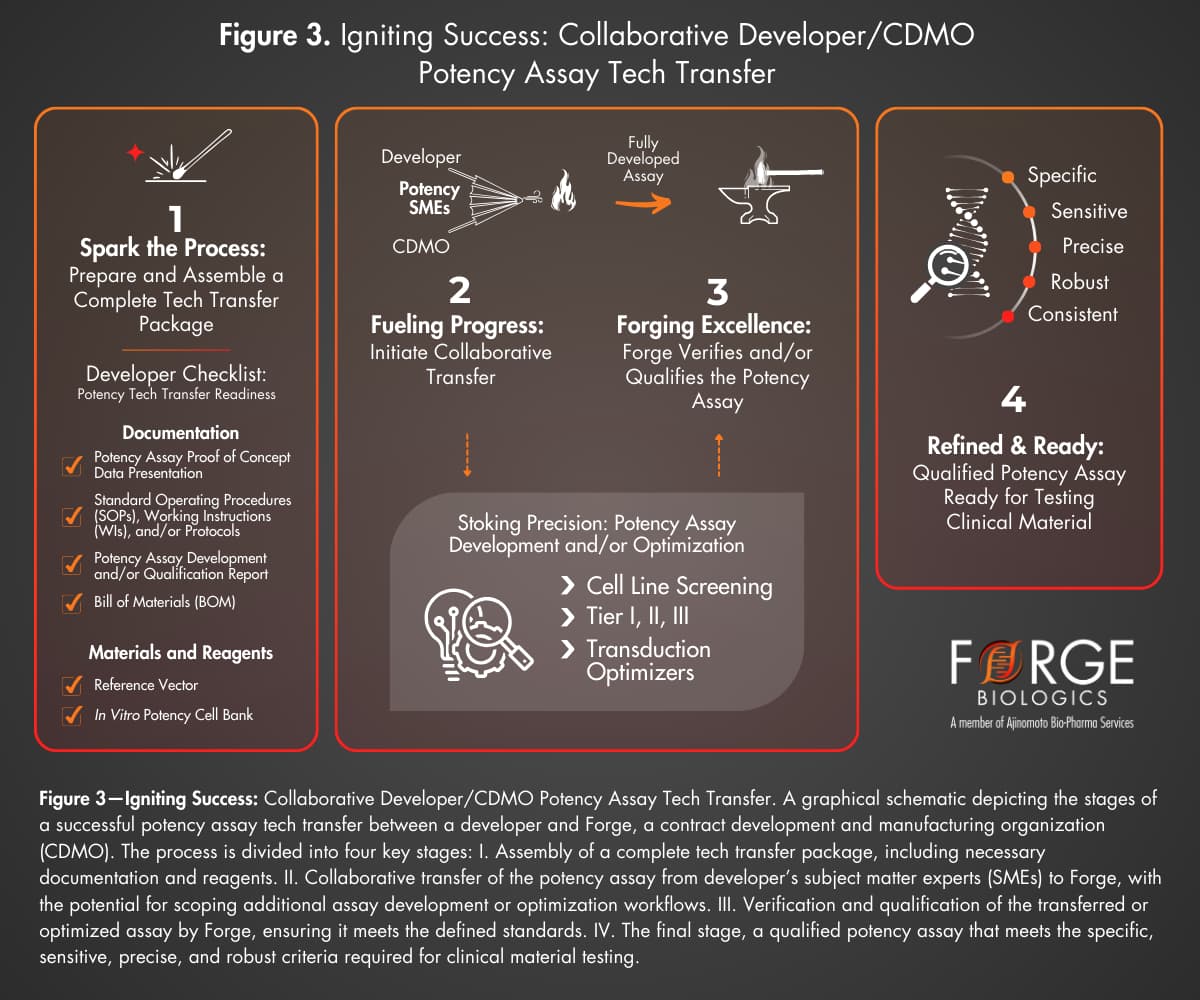
In addition to developing potency assays, Forge also facilitates the technology transfer of existing potency assays from developers to our AD/QC teams. This approach is often used for early-phase programs that have already developed Tier 1 or 2 potency assays for in-house characterization of lots. These assays can be transferred to Forge for qualification and eventual use in clinical batch release testing. Late-stage programs may transfer fully developed and qualified potency assays for use in release testing.
Effective technology transfer of potency methods requires comprehensive documentation and method verification to ensure accurate reproduction of assays in Forge’s facilities (Fig. 3). Developers contribute to a successful tech transfer by preparing a tech transfer package including finalized standard operating procedures (SOPs), work instructions (WIs), bill of materials (BOM), development and/or qualification reports (as applicable), and established specifications.
Developers are encouraged to provide reference vectors and/or cell banks, if available, to further accelerate verification. Clear communication and collaboration between developer SMEs and Forge’s potency team are essential for overcoming any challenges during the transfer process.
Conclusion
A sound strategy for evaluating in vitro potency of AAV gene therapies is essential for ensuring product consistency and meeting regulatory standards. Forge Biologics’ tiered approach provides a structured framework for potency assay development, enabling comprehensive evaluations of AAV potency across all phases. By leveraging advanced tools like qPCR, ddPCR, ELISA, JESSTM, and flow cytometry, Forge collaborates with developers to ensure the creation of reliable potency assays. As gene therapy continues to evolve, adopting advanced analytical methods and maintaining close ties with regulatory agencies will be key to overcoming the complexities of potency assay development and delivering effective therapies to patients.
References
- U.S. Food and Drug Administration. Guidance for Industry: Potency Tests for Cellular and Gene Therapy Products. 2011. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/potency-tests-cellular-and-gene-therapy-products. Accessed September 13, 2024.
- U.S. Food and Drug Administration. Human Gene Therapy Products Incorporating Human Genome Editing; Draft Guidance for Industry; Availability. Docket No. FDA-2023-D-4299, 2023. Available at: https://www.regulations.gov/docket/FDA-2023-D-4299/document. Accessed September 13, 2024.
- Ellis, B.L., Hirsch, M.L., Barker, J.C. et al.A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with Nine natural adeno-associated virus (AAV1-9) and one engineered adeno-associated virus serotype. Virol J 10, 74 (2013). https://doi.org/10.1186/1743-422X-10-74
Contributing Authors:
Samantha Powers, Ph.D., Scientist II, Analytical Development, Forge Biologics
Julia Zalewski, B.S., Associate Scientist II, Analytical Development, Forge Biologics