A new chapter in the decentralized clinical trial approach
Despite the promised decentralized trial revolution, we haven’t yet moved the needle in a significant way, although we are seeing far bolder commitments to this as we continue to experience the pandemic restrictions for some time to come. The vision of grandeur is one thing, but operationalizing and execution are another and recognising that change, particularly mid-flight on studies, is worthy of thorough evaluation and consideration in order to achieve success. Here we will discuss one of the critical building blocks of a Decentralized and Remote Trial strategy: TeleConsent; more than paper under glass, it is a paradigm change and key digital enabler.
As decentralized clinical trials have gained significant adoption in response to the global COVID-19 pandemic, there is a growing need for platforms that allow for TeleConsent to connect patients worldwide virtually with clinical sites. This allows clinical trial sponsors to screen, enroll, and consent participants in an environment where many patients are being told to shelter in place.
Traditionally, consent has been obtained using a paper process where the clinician and the patient are located in the same facility. Both parties sign the paper document to confirm it has been reviewed and understood. eConsent allows this process to be digitized and enables electronic signatures to be obtained at the site with both patient and clinician. This new process removes manual paper workflows and enables increased quality and compliance while checking real-time patient comprehension throughout the process.
TeleConsent, on the other hand, means that a patient can be located geographically anywhere, distant from the site, and follow the eConsent process. Patients can be in their own homes and connect virtually to the site by TeleVisit. This process can also be used in a “re-consent” for any future changes in a clinical trial. Like eConsent, it is crucial with TeleConsent that the system is in line with the parameters outlined by the FDA in their December 2016 guidance and 21 CFR Part 11, and ICH GCP E6 R2. In terms of security and privacy, the eConsent system should be aligned to HIPAA and GDPR.
Recently, we announced general availability of Medable TeleConsent™, a new product that enables fully remote informed consent and re-consent for clinical trials. Unlike traditional eConsent products that require both patient and investigator to be physically present together in the clinic, Medable TeleConsent allows patients, doctors, nurses, and clinical trial staff all to connect and sign remotely from any location.
Medable TeleConsent solves one of the most complex aspects of clinical trials for patients, sites and sponsors and defines the first experience for patients of trial participation. By eliminating the need for multiple round-trip visits to clinical sites, TeleConsent dramatically improves patient accessibility to studies from their home location connecting them directly with their PI’s and site teams resulting in faster enrollment and greater participant diversity for trial sponsors—and better retention over the course of a study as patients have more options to enhance understanding of trial expectations. TeleConsent also improves patient knowledge and comprehension by providing medical information in visual and multimedia formats, which patients can review in depth together with family members and caregivers—and then engage visually with their physician to sign consent forms digitally from the comfort of their home or local clinic.
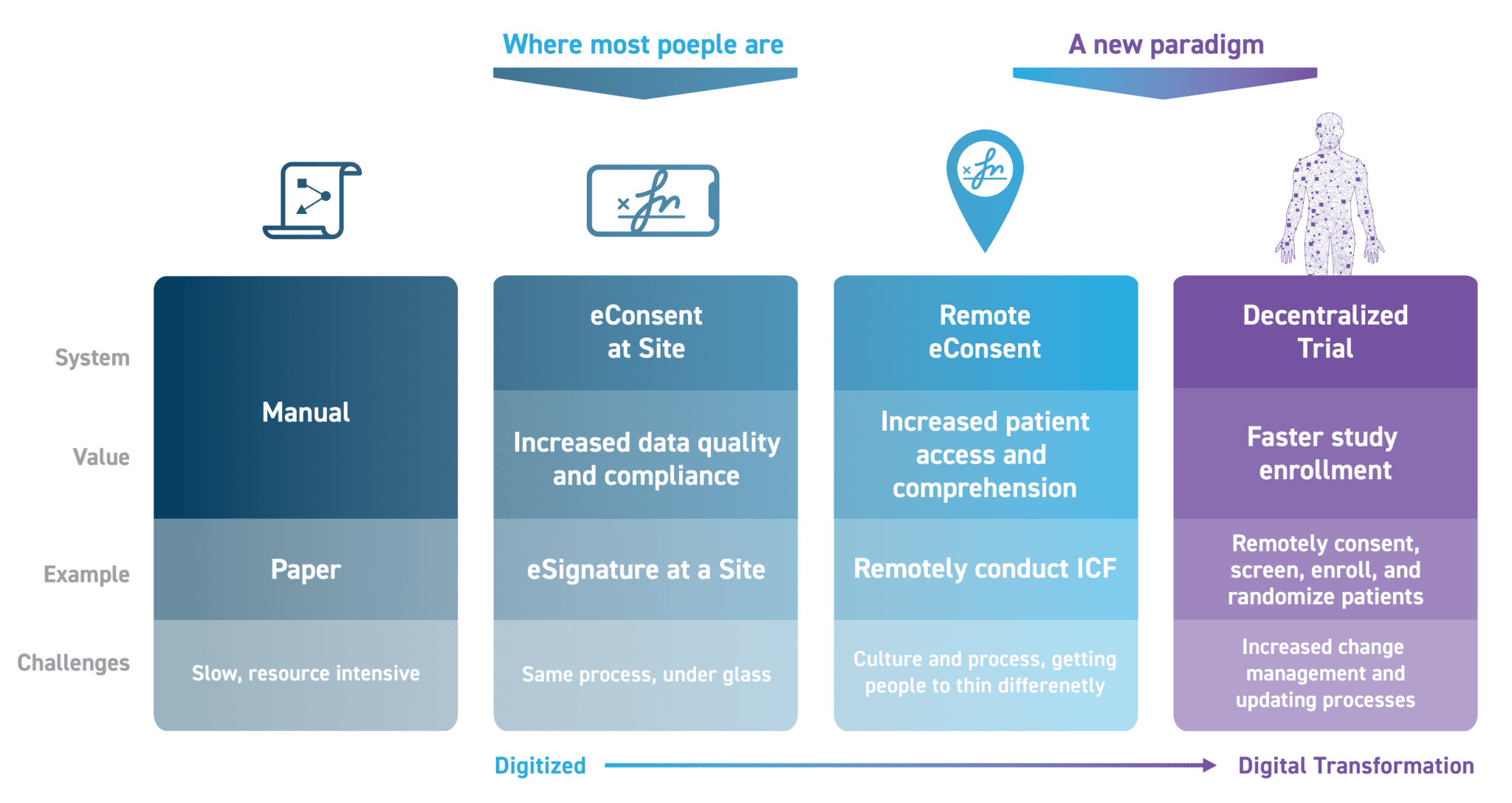
It’s important to note that eConsent operates on a continuum. With Medable TeleConsent, you can choose whether to utilize eConsent at the site, whether to complete the eConsent remotely, or whether to commit to a fully decentralized clinical trial where consent is not only remote, but so too is screening, enrollment, randomization, and supply. Figure 1 shows the journey toward full Decentralized Clinical Trial Informed Consents.
Medable TeleConsent is especially critical in the COVID-19 environment, where many patients are staying home to avoid social interaction and minimise exposure. Sites and sponsors can now screen, enroll and consent study participants without meeting in person, taking advantage of Medable’s TeleVisit application to conduct personalized interactions that improve patient understanding. Sites and sponsors benefit from streamlined workflows and enhanced data quality and compliance. Sponsors also get increased transparency with real-time reporting and insight into study progress. TeleConsent can also be used for re-consenting patients for any future changes that may happen in a clinical trial.
“Medable TeleConsent is a critical step in the evolution of decentralized research, as consent is the gateway to trial participation,” said Dr. Michelle Longmire, CEO and co-founder of Medable. “This has the potential to enable global patient access and improved knowledge, leading to greater participant diversity, retention, and ultimately research quality and speed. We’re excited to break down these barriers to benefit patients, sites and sponsors alike.”
To learn more about our TeleConsent, we invite you to schedule a TeleLunch and Learn where we’ll provide a live demo of our TeleVisit and TeleConsent capabilities.