
BYOD Best Practices: How Mobile Device Strategy Leads to More Patient-Centric Clinical Trials
Some of the most time- and cost-consuming components of clinical research center on gathering, analyzing, and reporting data. To improve efficiency, many clinical trial sponsors have shifted to electronic clinical outcome assessments (eCOA), including electronic patient-reported outcome (ePRO) tools.
In most cases, patients enter data using apps installed on provisioned devices. At a time when 81% of Americans own a smartphone, why not use the device they rely on every day?
Using patients’ mobile devices, as either a stand-alone solution to collect participant data or as part of a hybrid option that also incorporates provisioned devices, enhances patient centricity. That’s the theory behind the bring your own device (BYOD) strategy — a viable option for many clinical trials and a must for virtual or hybrid clinical trials.
While decentralized trial models and ePRO are both accepted in the industry, some sponsors have hesitated on BYOD due to questions over data quality, integrity, and variability; regulatory acceptance; and logistical issues. However, COVID-19 has prompted sponsors and CROs to give BYOD a longer look. Patients are less willing to travel to physical sites and more comfortable using their mobile devices in the context of healthcare. To encourage enrollment, sponsors must meet patients where they are: at home, on the go, or at the clinic.
With the FDA encouraging adoption of patient-centric approaches, clinical trial models that allow patients to report outcomes from home are the way forward. BYOD is a key component of this approach.
BYOD Benefits for Patients, Sponsors, and CROs
An increasing number of studies are deploying BYOD models with success, proving regulatory and data quality/consistency hurdles can be cleared. And the benefits to both patients and sponsors make BYOD a clear choice for many clinical trials.
The biggest win? Patients prefer it. The more seamlessly your clinical trial fits into patients’ lives, the more likely they will stay engaged. Think about it: Most smartphone users can’t part with their devices for five minutes. But if they left a provisioned device at the office, there it would stay until tomorrow. “I’ll enter my symptom info later,” they’d think. Not good for data accuracy.
A 2018 study explored patients’ preferences for paper-based and digital PROs. Patients completed a PRO using paper, a provisioned device, and an app installed on their mobile device. Patients later filled out a questionnaire about their attitudes toward each method.
Of the 155 participants, 94% said they would “definitely” or “probably” download an app onto their mobile device for a future clinical trial. A total of 45% thought BYOD would be more convenient. Only 15% preferred a provisioned device.
BYOD also paves the way for compliance. In an early Clinical Ink case study conducted on the Lunexis™ platform, patients leveraged the BYOD implementation twice as often and for twice as long (see below). This suggests patients were using the app as intended, which leads to better data and a smoother clinical trial.
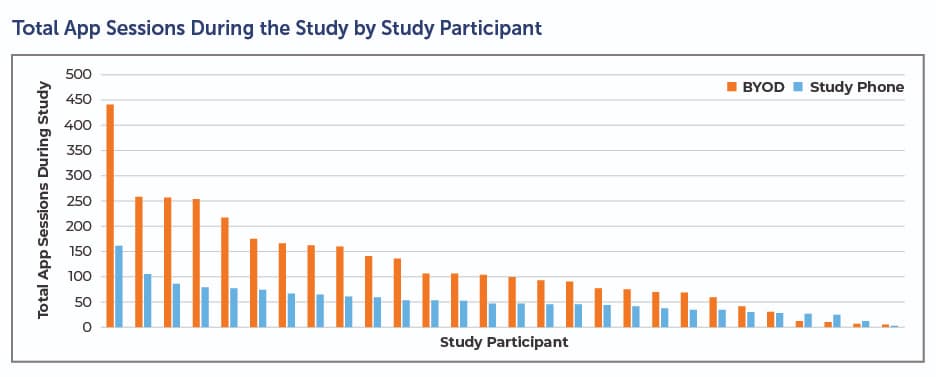
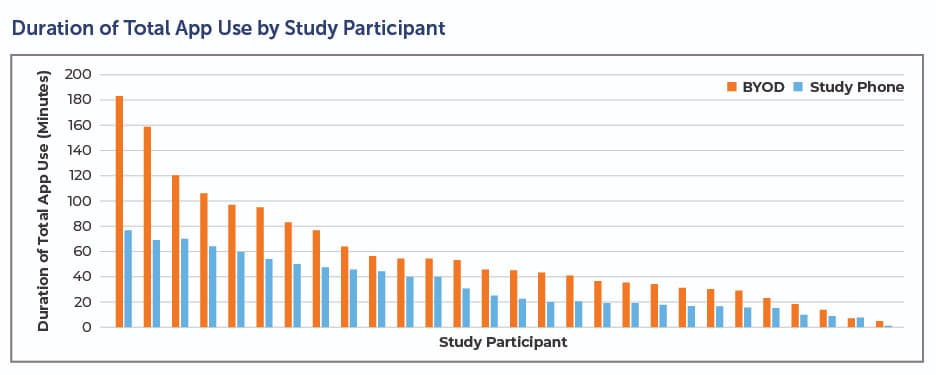
Clinical trial sponsors and CROs face enormous pressure to control costs and shorten timelines. BYOD helps achieve both. In addition to improving efficiency, BYOD significantly lessens — and perhaps eliminates in some trials — costs associated with data plans, shipping, lost or unreturned devices, and the overall logistics of inventory management. For a large trial, these savings are significant.
Regulatory Acceptance of BYOD Data
Multiple studies have completed regulatory submissions using BYOD-captured primary endpoint data. Concerning PRO assessments, in its COVID-19 guidance document, the FDA recommends “technologies that can remind trial participants to complete the questionnaires and/or verbal administration at the time instructed.”
This statement indicates FDA acceptance of mobile devices generally. Engage with the agency early in your protocol to get feedback regarding BYOD or a hybrid strategy.
Copyright Issues
Any approach for BYOD should be reviewed and approved by the ePRO questionnaire copyright holders before ePRO development begins. If the copyright holder has expressed comfort with BYOD or if the questionnaire was created by the sponsor or CRO, you’ve got the green light for BYOD.
As you plan ePRO development, raise any copyright concerns with your vendor. They may have relationships that can help facilitate the process.
Data Equivalency
Multiple studies and meta-analyses show high levels of agreement between paper and electronic formats, as well as between provisioned devices and patient smartphones. If you follow general principles of ePRO design good practices, measurement capabilities between paper-based data collection and mobile devices should be equivalent.
The BYOD Bottom Line
BYOD is an important component of patient-centric clinical trials. The approach doesn’t suit every trial — some may require a hybrid approach while others may require provisioned devices exclusively. To determine whether BYOD is right for your or your client’s trial, consult with a BYOD-experienced vendor to evaluate risks and opportunities and determine the best path forward.