Clinical trial monitoring is about to change forever
In 2006, a clinical trial called ILLUMINATE halted abruptly. Trial subjects treated with an investigational drug called torcetrapib started experiencing unexpected cardiovascular problems, in some cases resulting in death. After 15 years and nearly 1 billion dollars, the development of torcetrapib froze.
Nine years later, a paper published in the journal Circulation explained how the ILLUMINATE disaster could have been predicted and prevented if just 9 proteins had been measured in clinical trial participants following initial treatments.
The challenge and promise of proteomics
There is one universal truth when it comes to disease pathology, no matter what kind of disease we’re talking about: proteins change. Whether by shifting patterns of expression, post-translational modifications, intra and extra-cellular transport, or – usually – all of the above, when health status changes, proteins change. The question is, which proteins?

All of the news, delivered with full-text to your inbox. For professionals discovering, developing, and marketing biopharmaceutical drugs.
For the past 20 years, SomaLogicTM has strived to simultaneously answer this question and make it irrelevant. If you don’t know which proteins to look at, the obvious approach is to assay all of them, or as many as technologically possible. For that, we built the SomaScan® Assay, a proteomics platform that leverages modified aptamers for near limitless multiplexing capabilities.
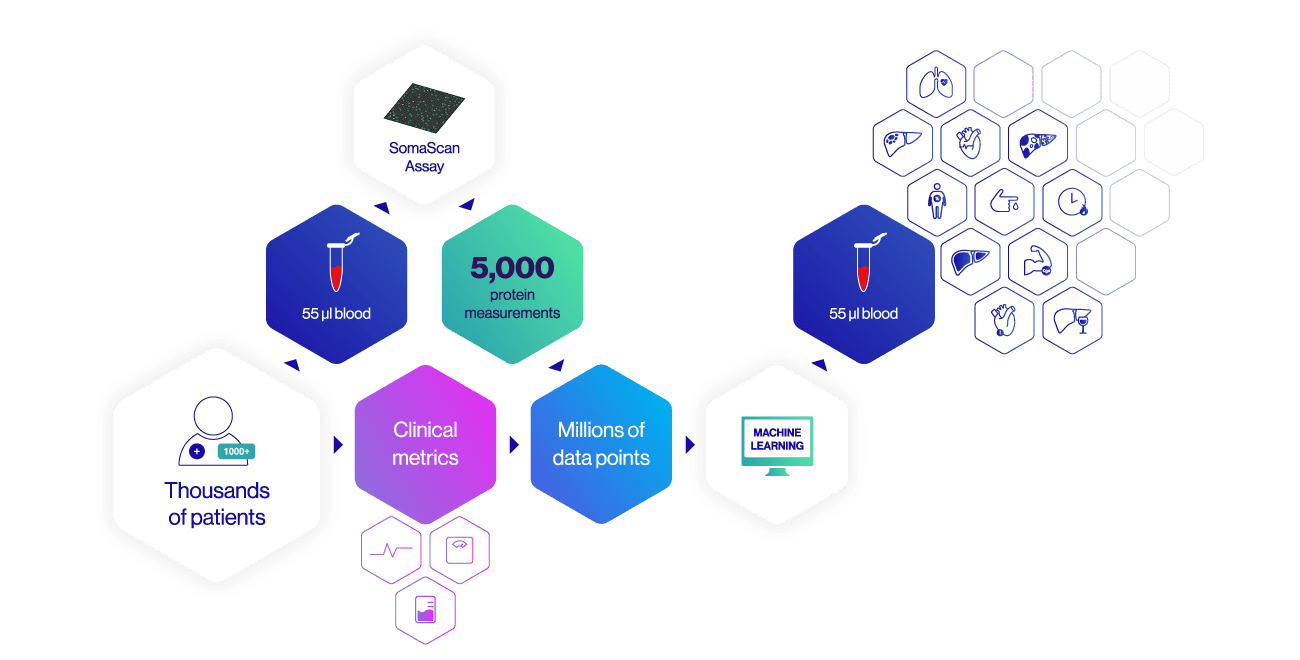
The first iteration of the SomaScan Assay measured 400 proteins. We quickly grew that number to 800, 1,030, 1,129, 1,305, and now 5,000. At 5,000 proteins, the SomaScan Assay can deliver 250% more protein measurements than any other high-throughput proteomics platform on the market, and it’s poised to expand.
What we built in the SomaScan Assay, is an incredibly powerful tool for protein biomarker discovery. Along with our many academic and industry collaborators, we’ve extensively analyzed SomaScan data in a variety of patient groups in order to identify which protein signatures are most relevant for a given clinical metric. The result is our newly launched SomaSignal™ Tests.
From proteomic data to clinical metric
SomaSignal Tests are developed by analyzing thousands of proteins in thousands of patients using the SomaScan Assay. Protein signatures revealed by the SomaScan Assay are compared against standard clinical metrics in order to identify patterns of protein changes that correlate with current health status and future trajectory using machine learning.
Each pattern becomes the basis for a specific SomaSignal Test, of which there are currently 18. Two of those tests, which predict primary and secondary cardiovascular risk, were developed from further investigation of the 9 proteins that were critical in the ILLUMINATE trial.
Because each SomaSignal test is generated using data from subsets of the same 5,000 proteins, results for all the SomaSignal tests can be derived from a single 55µl blood sample. Eighteen tests is the first step towards a comprehensive liquid health check: with many more in our development pipeline.
Moving towards a comprehensive liquid health check
The development of the SomaSignal Tests is systematic and founded on a body of peer-reviewed research. As a result, the menu of tests today is largely focused on a few disease/health areas. We have tests for heart disease/failure risk, liver health, including non-invasive indicators for non-alcoholic steatohepatitis, and tests relating to metabolic health and diabetes, such as blood glucose tolerance and body fat.
We make a few well-defined and well-supported claims when it comes to the SomaSignal Tests. You can use them to stratify patients, predict risk, monitor treatment efficacy, and gain insights into mechanisms of action, using 18 different clinical metrics, all from a single blood sample.
Today, the SomaSignal Tests can help improve and increase the speed of clinical trials and make them safer for participants. Tomorrow, we’ll continue expanding towards that vision by building a suite of clinical research tests based on a foundation of data and 5,000 proteins (and counting).
We are working now to develop tests for additional applications, such as neurological health, autoimmune diseases, cancer, infectious diseases, as well as healthy sleep, fitness, nutrition, and aging. But we need your help. Collaborate with us and help us identify protein signatures for new disease areas. Use our tests in your clinical trials to find the right patients faster and characterize and monitor them in ways that were previously unavailable.