Conquering a silent killer: HDV and Eiger BioPharmaceuticals
Hepatitis delta, also known as hepatitis D, is a liver infection caused by the hepatitis delta virus (HDV) that results in the most severe form of human viral hepatitis for which there is no approved therapy.
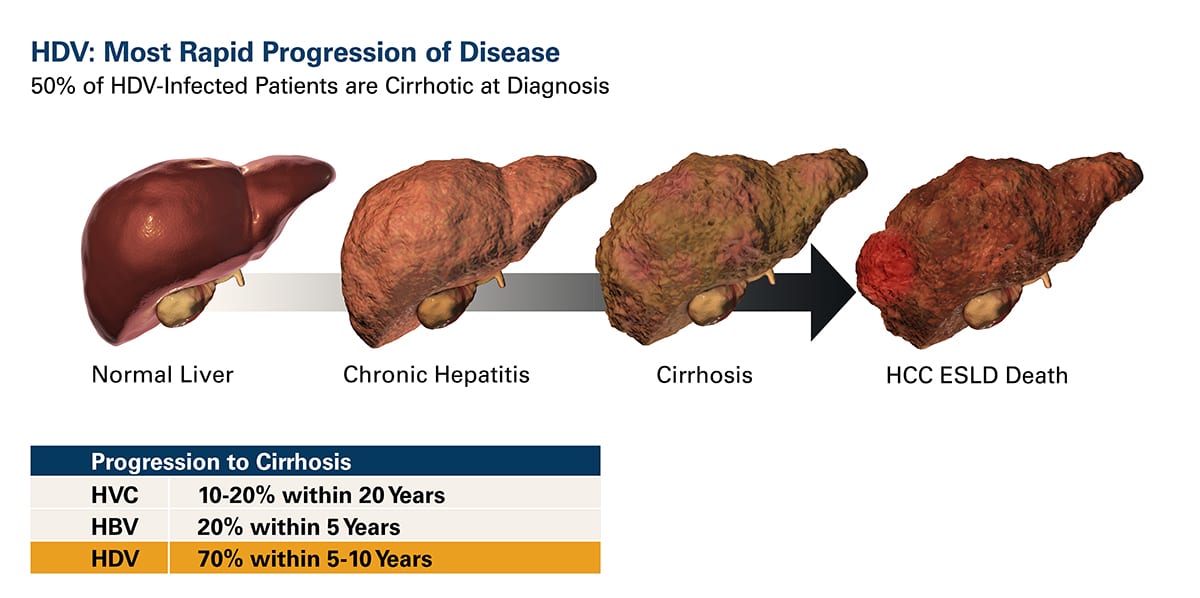
HDV is a single-stranded, circular RNA virus that requires the envelope protein (HBsAg) of the hepatitis B virus (HBV) for its own assembly. As a result, hepatitis delta virus (HDV) infection occurs only as a co-infection in individuals infected with HBV. However, HDV/HBV co-infections lead to more serious liver disease than HBV infection alone. HDV is associated with faster progression to liver fibrosis (progressing to cirrhosis in about 80% of individuals in 5-10 years), increased risk of liver cancer, and early decompensated cirrhosis and liver failure.
HDV is the most severe form of viral hepatitis with no approved treatment.
Approved nucleos(t)ide treatments for HBV only suppress HBV DNA, do not appreciably impact HBsAg and have no impact on HDV. Investigational agents in development for HBV target multiple new mechanisms. Aspirations are high, but a functional cure for HBV has not been achieved nor is one anticipated in the forseeable future. Without clearance of HBsAg, anti-HBV investigational treatments are not expected to impact the deadly course of HDV infection anytime soon.
HDV is a large and growing Orphan Disease in the western world.
Worldwide, an estimated 15-20 million people are infected with HDV, approximately 6% of the HBV-infected population. HDV is historically most prevalent in the Middle East, Asia, and Africa. However, migration from regions of high prevalence in the last decade has increased HDV prevalence in the Western world, becoming a large healthcare concern with infected individuals estimates of ~100,000 in the U.S. and ~200,000 in Western Europe. There is no vaccine for HDV, and once a patient is infected with HBV, the hepatitis B vaccine will not prevent infection with HDV. Therapies are urgently needed.
HDV has become a target for drug developers, leading to potential therapies to attack the silent killer.
Eiger BioPharmaceuticals is a late-stage biopharmaceutical company focused on the development and commercialization of treatments for serious rare and ultra-rare diseases and is leading the charge in the development of treatments for HDV.
Eiger is targeting HDV infection with two FDA Breakthrough Therapy Designation programs: Lonafarnib and Peginterferon lambda (Lambda).
Lonafarnib is the only oral agent in development for the treatment of HDV.
Lonafarnib is a well-characterized, first-in-class, orally active inhibitor of farnesyl transferase, an enzyme which attaches a lipid chain to proteins through a host process called prenylation. HDV uses this host cell process inside liver cells to complete a key step in its life cycle. Lonafarnib inhibits the prenylation step of HDV replication inside liver cells and blocks a critical step in the virus life cycle. Lonafarnib is currently in Phase 3 for the treatment of HDV with a single, pivotal, international trial called D-LIVR. The D-LIVR study (n=400, anticipated enrollment) is ongoing, activating sites and enrolling patients. D-LIVR is the first-ever global Phase 3 HDV study and will span twenty countries with over one hundred sites.
Lonafarnib has been granted Orphan Drug Designation by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA), Fast Track Designation and Breakthrough Therapy Designation by U.S. FDA and PRIME designation by the EMA. Lonafarnib is not approved for any indication.
Lambda may be a better tolerated interferon in development for the treatment of HDV.
Peginterferon lambda (Lambda) is a well-characterized, late-stage, first-in-class, type III interferon (IFN) that stimulates immune responses that are critical for the development of host protection during viral infections. Lambda is being developed as a better tolerated interferon with fewer of the typical flu-like, depression and blood count lowering side effects associated with other interferons. Lambda has previously been administered to over 3,000 patients in Phase 1, 2, and 3 clinical trials for HBV and HCV, and has a large, well-understood safety database. Eiger has investigated Lambda in over 50 HDV-infected patients across international academic centers in two Phase 2 studies.
Phase 2 LIMT Lambda monotherapy study results demonstrated durable suppression of HDV RNA in 36% of patients at 24 weeks post-treatment. Phase 2 LIFT Lambda / Lonafarnib combination study interim results were recently reported at The LIVER meeting (AASLD 2019), with >50% of patients HDV RNA suppressed to unquantifiable levels at Week 24 and 95% of patients achieving the primary endpoint of > 2 log decline in HDV RNA at Week 24. Eiger plans to advance Lambda in Phase 3 registration for HDV in 2020.
Lambda has been granted Orphan Drug Designation by the U.S. FDA and EMA, and Breakthrough Therapy and Fast Track designation by U.S. FDA. Lambda has not yet been approved for any indication.
Lonafarnib and Lambda hold promise as future foundational treatments for patients with HDV infection.
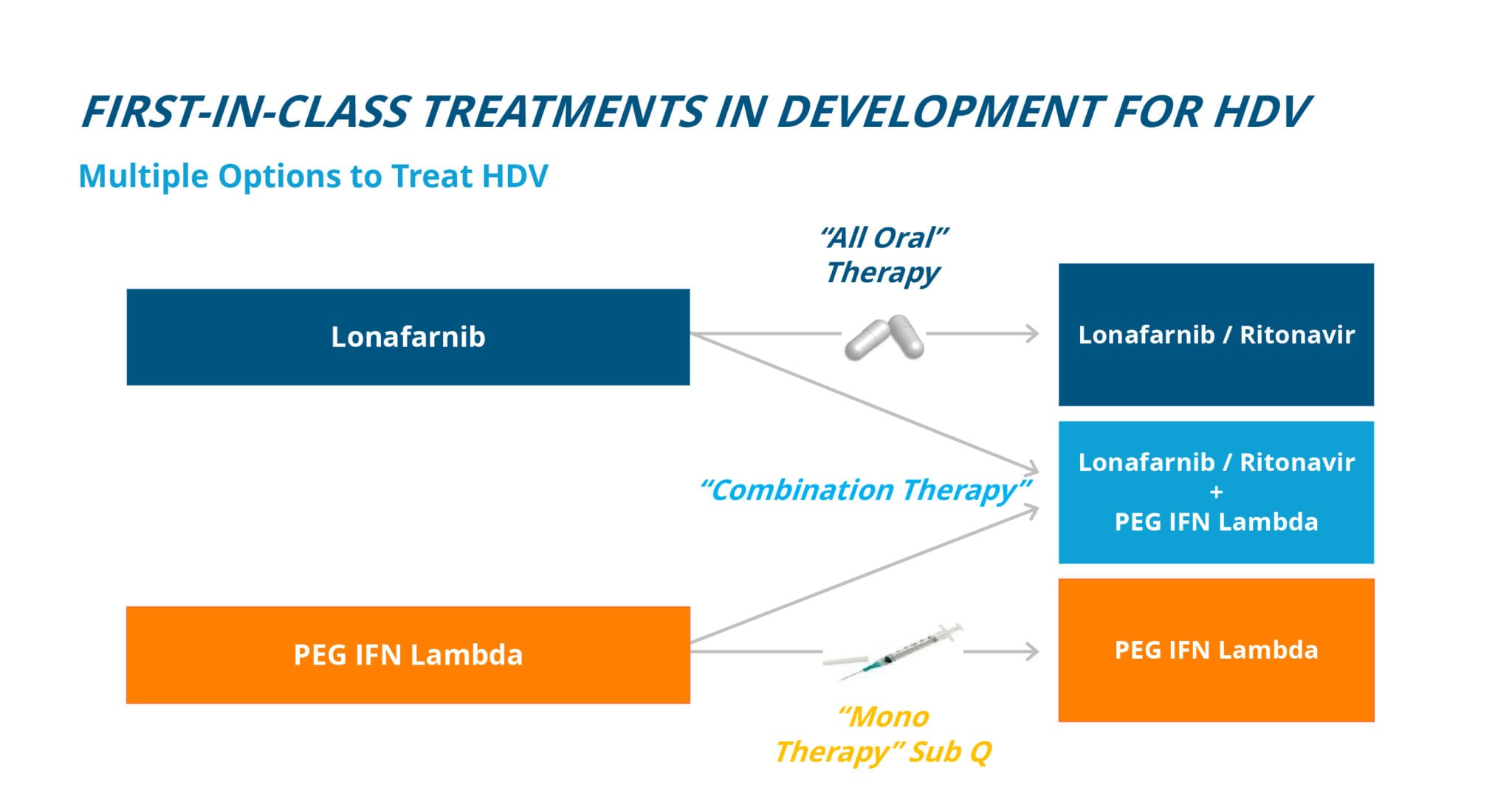
Attacking the HDV virus with multiple therapies, targeting different mechanisms of action to disrupt the virus at different points in the viral life cycle, is expected to yield the most robust results toward a future HDV cure. Lonafarnib and Lambda are expected to be foundational therapies for future treatment, alone or in combination, with other investigational treatments for HDV.
HDV Awareness and Testing
It is important that patients with chronic HBV get tested for HDV. There is now a simple and easily accessible blood test for HDV from Quest Diagnostics in the U.S. Testing for HDV may help save lives by insuring co-infected individuals are diagnosed and receive appropriate medical care and treatment as early in the disease course as possible.
Eiger is sponsoring a Hepatitis Delta Testing Program whereby healthcare practitioners are invited to submit HBV-positive patient blood samples to Quest Diagnostics for HDV testing. Eiger covers the costs of blood draw, test, and test reporting.