
Critical Junctures in Gene Therapy Clinical Material Manufacturing: Where Do I Start? And Will It Work?
Strategic approaches to successful adeno-associated virus (AAV) manufacturing campaigns often include robust and early analytical characterization, as well as consistent, data-driven re-evaluation of manufacturing plans throughout a program’s lifecycle. Holistic product development of therapeutics utilizing AAV vectors requires the synergistic consideration of pre-clinical or clinical development plans overlayed with a thoughtful AAV manufacturing strategy. Both product development and manufacturing will require an indication-centric and patient-centric approach. However, AAV manufacturing programs must also accommodate material needs, study timelines, and product-specific requirements, often dictated by the route of administration, intended dose, potency, buffer compatibility, and tissue/organ sensitivity to the product. While material need and availability may be quickly predicted based on yield projections for the chosen serotype and study design, positioning an AAV manufacturing program for success also requires an understanding of the AAV product itself and potential process adjustments that may enable greater success. At Forge Biologics, we leverage our bespoke platform process coupled with Drug Master Files for the Forge AAV manufacturing process and plasmid designs and manufacturing strategies, which provide our clients an adaptable starting point in developing their manufacturing process. Our extensive experience in manufacturing natural and novel AAV serotypes using our platform technologies allows accelerated transition from small-scale pilot production through cGMP Clinical Trial Material (CTM) production. With numerous development studies, scales, and grades of AAV available to build manufacturing pathways that will successfully reach program milestones, a common two-fold question remains: ‘Where do I start, and will it work?’
While the starting point for most manufacturing programs will be a small-scale platform pilot run, the learnings from this pilot run may inform a labyrinth of next steps. To establish the vast scope of optionality available when pursuing product-specific process development, our technical teams have compiled a list of more than fifty studies that may be pursued to optimize drug substance and drug product manufacturing processes. Optimizing yield—or increasing purity of the final product—is often essential to long-term program feasibility, but interestingly, two of the most impactful strategies to guide success are independent of product, serotype, therapeutic indication, or intended manufacturing scale. Below, we investigate the high-powered strategies that provide programs a foundation for success by, 1) building a program around robust early analytics, and 2) continuous re-evaluation of your manufacturing approach as data becomes available.
Building a Program with Robust Early Analytics
Where do I start?
To begin designing a program that will generate a robust analytical characterization package for regulatory filings, the quality target product profile (QTPP) is first drafted for the product. The QTPP, as defined by ICH Q8(R2), is ‘a prospective summary of the quality characteristics of a drug product that ideally will be achieved to ensure the desired quality, taking into account safety and efficacy of the drug product’¹. This document will outline the target characteristics and clinical requirements for the therapeutic product, such as the route of administration, dose strength, and product stability, thus providing the foundation for identifying quality attributes (QAs). QAs can then be assessed for their potential impact to the product in order to determine preliminary critical quality attributes (CQAs). CQAs are defined as physical, chemical, biological, or microbiological properties or characteristics that should be within an appropriate limit, range or distribution to ensure the desired product quality¹. CQAs are typically broken into characteristics that relate to the purity, safety, quantity, identity, and potency of the drug product. For AAV, example CQAs may include physical titer, infectious titer, and endotoxin levels, as an out-of-range result of any single characteristic may negatively impact product quality or safety. After a list of CQAs with projected target ranges that comply with the guidelines in the QTPP have been generated, an assay panel suitable to evaluate the process and product can be defined.
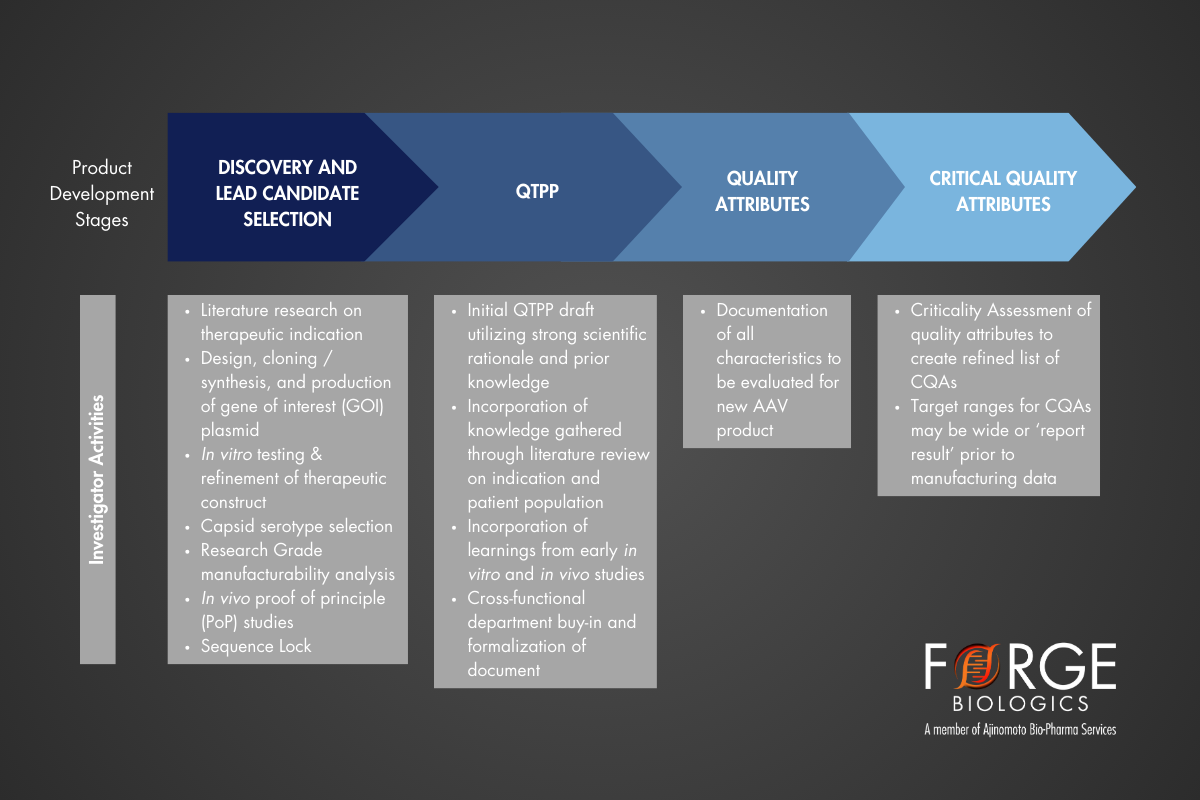
In practice, aligning on a QTPP and CQAs for a yet to be manufactured AAV product is a difficult task, and often takes a retroactive route. An early draft may represent a brief, idealized product profile, with an understanding that continuous revisions will be made throughout the program lifecycle. Defining the ideal criteria for the product is the starting point for ensuring early assay panels provide sufficient insight to support or justify revision of your CQA targets.
Will it work?
With the QTPP and CQAs in place, robust assay panels—inclusive of a phase-appropriate potency assay² for product characterization—will further help direct a pathway to cGMP manufacturing readiness. From the first production run onward, thoughtful assay panel design allows data-driven refinement of CQAs, with the goal of understanding the link between potency and process. Narrowing CQA target ranges and reducing the number for CQAs is an expectation of program maturity and process control as the program progresses to later clinical and commercial stages. Characterizing in-process and final samples from early production lots can provide insight to guide development studies that are narrow in scope, rather than post-hoc analyses which can lead to time-consuming investigation efforts. With the goal of developing a controlled AAV process and consistent product, robust early analytics poises a program for success by utilizing each production batch to gain valuable insight.
Continuous Re-Evaluation of Manufacturing Approach
Where do I start?
Now that the AAV product development pathway and goals are clearly defined by the QTPP, CQAs, and analytical plan, the program has an established baseline to measure success. Continuous re-evaluation of the manufacturing pathway for an AAV product begins with building a strong and adaptable team. A well-rounded team capable of organizing data, interpreting results, critically thinking, and redirecting when required, may consist of professionals spanning program management, technical program strategy, and regulatory affairs, as well as manufacturing subject matter experts. A common misconception is that the manufacturing pathway is inflexible after program initiation. However, program changes and realignments should be viewed as continuous improvements, applied after learning more about the AAV process and product. To this end, after each production run, data should be compiled and evaluated meticulously against the QTPP, desired CQA targets, and future material needs. Utilizing a process information management software (PIMS) or creating an in-house solution to data organization is key for efficient decision-making and program evaluation. Additionally, incorporating at-scale manufacturing runs will generate the most relevant data to better understand the inherent variability of your product.
Will it work?
With a cross-functional team of experts, ideally with representation from the product sponsor as well as the manufacturing partner, consistent re-evaluation of data and program strategy will provide benefit to any therapeutic development program in the following ways:
- CQA targets can be evaluated for feasibility and proactively adjusted as needed for subsequent manufacturing runs, with the goal of achievable and accurate targets in place for a Toxicology or cGMP run.
- CQA target ranges can be narrowed and refined to reflect process and product understanding, as well as program maturity—a requirement for later stage clinical investigations and commercialization.
- Additional process development needs can be identified early in the manufacturing scale-up pathway to preserve the applicability of subsequent data.
- Earlier alignment in manufacturing scale adjustments, or recommended studies to achieve material needs can be proactively implemented.
Case Study
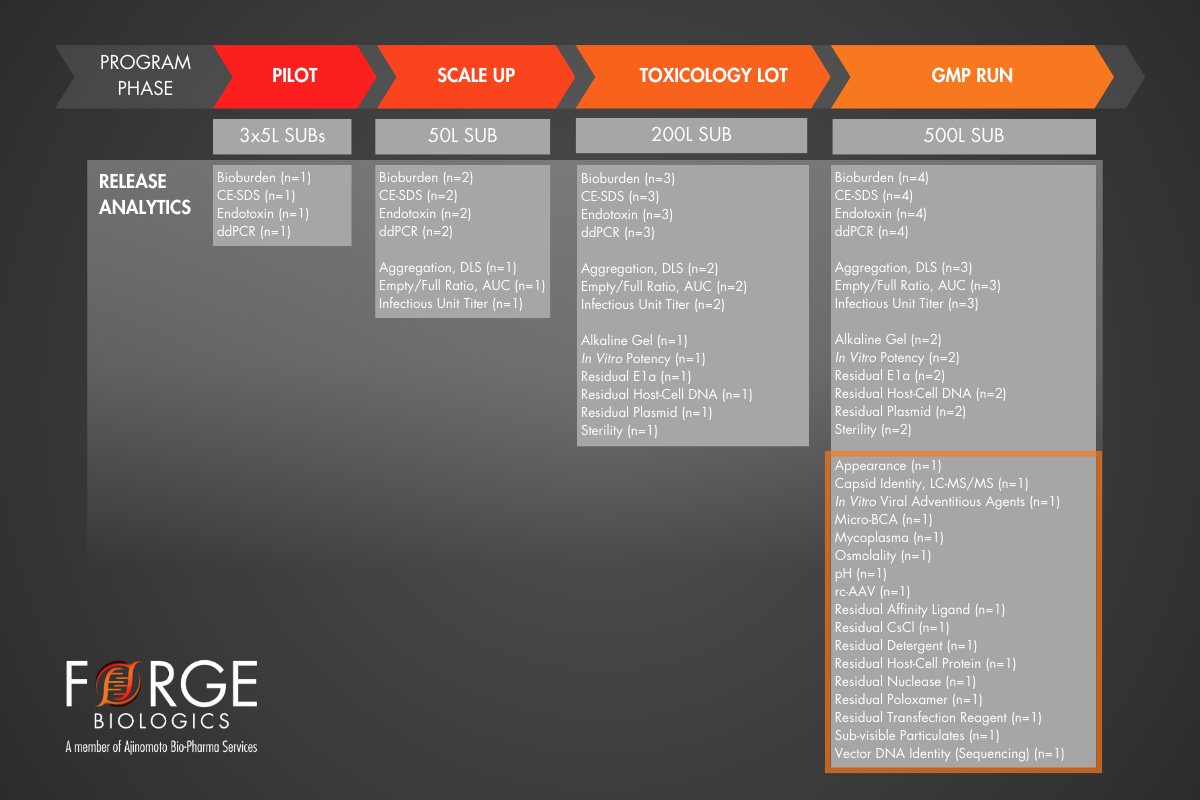
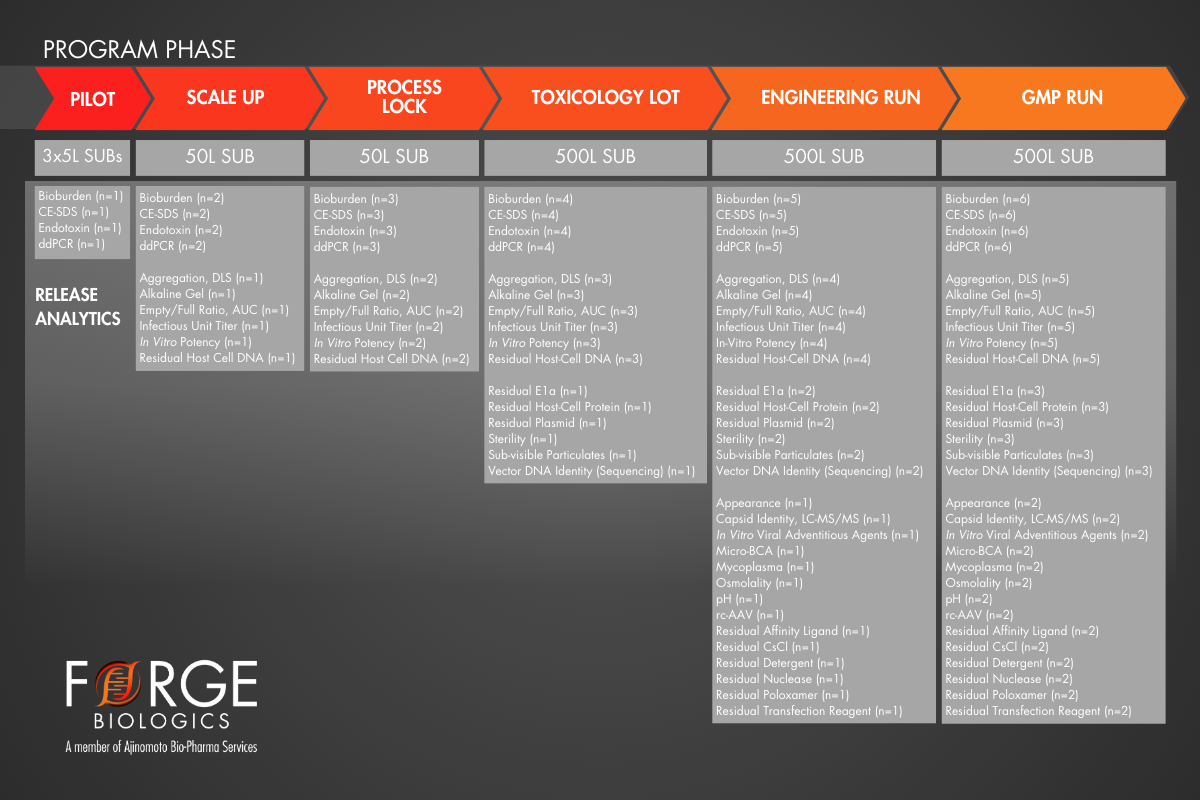
To explore the benefits of robust product characterization early in the manufacturing pathway, the example below outlines a case study featuring two programs forging different paths to arrive at cGMP Clinical Trial Material. In this case, Program 1 utilized a very common approach of the minimum viable path, which is suitable for high yielding, readily manufacturable products. However, Program 1 entered a cGMP manufacturing campaign without data on several critical residual assays, risking the overall success of the campaign. On the other hand, Program 2 invested in early AAV process and product understanding at small scale, as well as a full-scale engineering run. Program 2 employed a more risk-adverse approach, which may have been prompted by early results in conflict with the goals outlined in their QTPP. Both programs utilized the same analytical methods for cGMP CTM release; however, Program 2’s approach provided increased batch history and replicate data, which subsequently increased statistical power to narrow specification ranges that would achieve the goals of their QTPP.
Case Study: Program 1
Case Study: Program 2
Defining Program Success Criteria Prospectively
At the outset of a new AAV therapeutics program, drafting success criteria, an analytics plan, and assembling a versatile team for consistent program evaluation will establish a strong foundation that may withstand common pressures encountered along the development pathway. With these strategies for success in place, events such as unanticipated results or shifting deadlines can be met with rational and data-driven program adjustments. Preparing program requirements before manufacturing data is available is a daunting task, but the benefits of creating a baseline against which to measure success should not be overlooked. Utilizing resources such as academic literature, information on the indication or patient natural history, or knowledge from a platform manufacturing process that has encountered many product types, may allow a QTPP to be established which can be further honed throughout the product development journey.
Time, risk, and cost are often in opposition when it comes to creating an AAV manufacturing pathway. On one extreme, the most risk adverse plan will be the least cost- and time-efficient. On the other extreme, the most cost- and time-efficient plan often carries most risk for the program sponsor. To balance this, an intermediate approach to generate a sufficient amount of data without surpassing acceptable deadlines or budget, is a common way to balance risk, success, and patient safety. Given that most AAV gene therapies are currently endeavoring to create treatment options for patients with rare, and often fatal diseases, the greatest risk may be not reaching the clinic at all. To be a good partner to program sponsors and the patient communities they serve, Forge provides flexibility in manufacturing solutions and client-focused teams that can be tailored to the requirements of each individual program. With a strong strategy in place from the outset, when challenges arise, ‘There is always a solution.’
Additional contributions from:
- Angela Coy, Ph.D., Senior Manager, Regulatory Affairs
- Julianne Bartz, Manager, Regulatory Affairs
References
1 ICH Q8(R2) https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf
2 U.S. Department of Health and Human Services. Food & Drug Administration. Center for Biologics Evaluation and Research. December 2023. (Draft) Potency Assurance for Cellular and Gene Therapy Products. https://www.fda.gov/media/175132/download