
De-risking the patient journey in cell therapies: Six key considerations
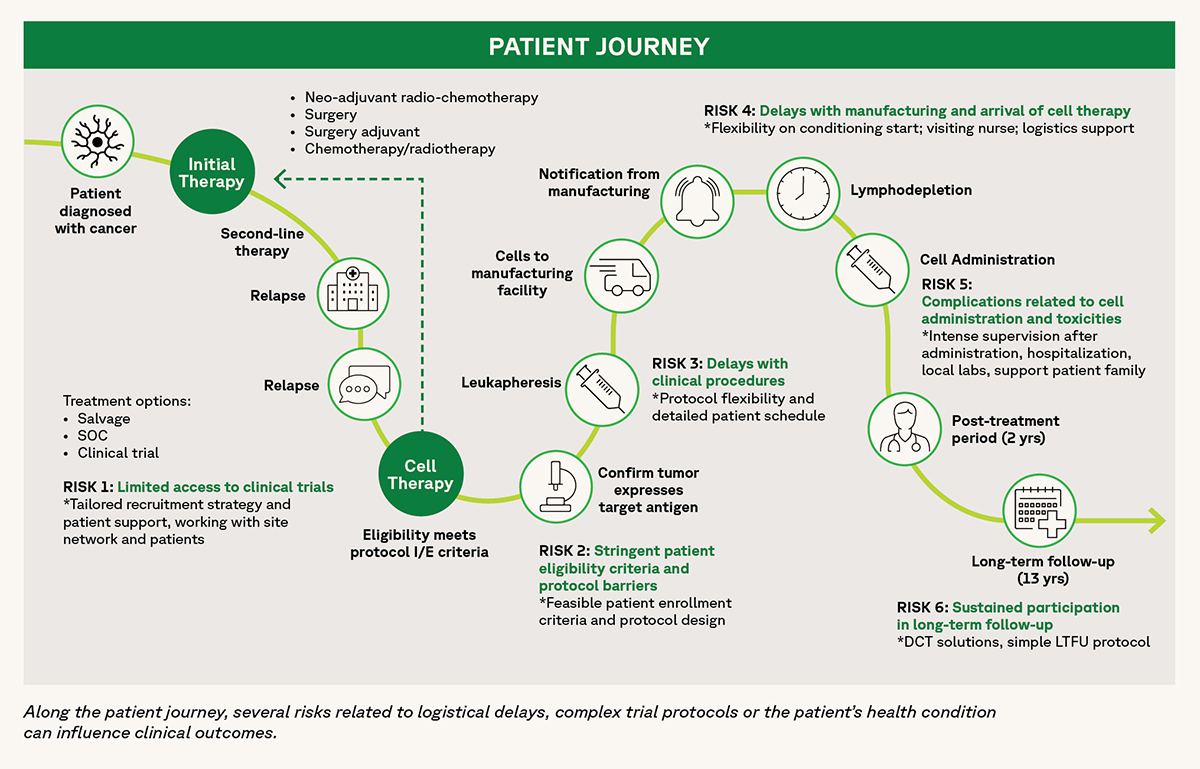
Successfully completing a cell therapy program (CAR T, TCR, TIL or other cell constructs) isn’t without inherent risks or unexpected delays. The patient’s needs, the physician’s guidance, healthcare limitations and logistical challenges are only a few of the factors that need to be considered. At any step, from vein to vein, unidentified risks or unanticipated time delays can significantly impact the patient’s clinical journey. To run a timely and hurdle-free cell therapy program, de-risking strategies need to be built into the action plan.
From patient screening, through cell product infusion and to long-term follow-up, the patient journey has numerous risks that can derail clinical progress. As Fortrea is committed to facilitating the best possible clinical outcomes, years of supporting and designing dozens of cell therapy programs, including all 6 FDA-approved CAR T-cell therapies, have led to our identification of six risk nodes along the patient journey. In this paper, we present these six risk nodes, as well as describe actionable measures designed to mitigate each of these risks.
6 risk nodes
In this paper, we present these six risk nodes, as well as describe actionable measures designed to mitigate each of these risks.
Risk 1: Limited access to clinical trials
How we address this:
Making clinical trial participation more accessible to all patients is one of our primary goals. By partnering with the Sponsor, we make trials available and visible to potential patients in countries participating in the trial but also outside countries involved. To improve trial visibility and encourage participation, we’ve partnered with direct-to-patient support organizations and patient advocacy groups. Through these efforts, we’re able to highlight clinical trial opportunities to patients already diagnosed with the disease of interest as well as educate them on options for better clinical outcomes.
Fortrea experts are always keen to partner with a Sponsor and design the trial protocol, taking into account the voice of the patient and prioritizing the patient’s well-being and health. Available data on patient preferences and the needs of their caregivers are taken into account to minimize patient burden. To make participation easier during the trial, we aim to offer decentralized trial support through technology-enabled solutions where possible.
Additionally, we partner with leading established cancer centers with proven expertise in cell therapies to ensure that clinical trials are aptly placed at sites equipped with the capabilities and clinical know-how to provide every patient with a safe and stress-free experience.
Risk 2: Stringent patient eligibility criteria and protocol barriers
How we address this:
Patient eligibility criteria has a direct impact on patient enrollment. Getting the balance right on how stringent enrollment needs to be requires careful attention to its design – too stringent, and enrollment is impacted; too lenient and regulatory requirements are not likely to be met. Based on the needs of the sponsor, our team of medical consultants and experienced protocol writers can design protocols with feasible patient enrollment criteria while fully upholding regulatory requirements and goals of the trial.
While Fortrea’s expert team always tries to be involved in protocol design and finalization, we review inclusion-exclusion criteria and identify potential enrollment barriers, such as molecular genotyping, screening for specific tumor-associated antigens or patient age restrictions. We also carefully examine the protocol language as this can potentially pose restrictions or expand timelines, for example, limiting where lab tests can be performed. Adding elements of flexibility to clinical trial protocols before finalizing them eliminates artificially imposed barriers, reduces number of protocol deviations and significantly improves patient enrollment and patient trial experience.
Before a trial begins, we ensure that the site is ready and fully set up to execute the cell therapy clinical trial protocol. During the trial, we assist sites with operationalizing these protocols most optimally, often providing guidance to help mitigate risks or delays. All members of the multidisciplinary site team are trained in trial procedures, the apheretic center is properly qualified, and cell therapy logistic pathway is established and validated by the Fortrea team.
Additionally, our medical team is closely involved in producing educational materials to provide patients and their families/caregivers with up-to-date information regarding the trial process, timelines, and potential side effects and complications. These information sheets and videos are made available to the patients along with the informed consent forms.
Risk 3: Delays with clinical procedures (such as apheresis)
How we address this:
Once patients are identified at the clinical trial sites, an active discussion between the site, the sponsor and the Fortrea clinical team begins. Details regarding the patient’s current status and availability, screening, progress with signing consent forms, timing of trial procedures such as cell collection and so on are tracked and reported to the sponsor’s teams sometimes in real time instead of weekly meetings. Questions arising at the trial site regarding patient assessment or procedure scheduling are promptly answered by Fortrea’s medical monitor and clinical team.
We ensure that flexibilities available in the protocol are fully exercised to get the patients through the process without unnecessary delays and barriers. If unexpected delays are anticipated, the dedicated Fortrea team immediately notifies the sponsor so contingency plans can be executed to address risks and ensure the patient can be successfully treated. Meanwhile, the attending physician manages the patient’s health so eligibility criteria can still be maintained.
Risk 4: Delays with manufacturing and arrival of cell therapy
How we address this:
Our Fortrea logistics coordinator remains in regular contact with the manufacturer and logistic vendors, receiving updates on schedules, backlogs or delays. In addition to the personal communication by the coordinator, our partnership with TrakCel offers a technological advantage in staying updated. Every package holding the patient’s cells is geo-tagged and tracked through the shipping and manufacturing cycles, its status updating every 15 seconds.
If delays are noticed or quality concerns become apparent, the sponsors and the clinical sites are immediately notified so that physicians can start discussing possible bridging therapies, as allowed by the trial protocol, for patients before cell therapy product becomes ready for infusion.
As we support ongoing cell therapy clinical trials, we’re also gathering real-world evidence about quality attributes and optimum logistical practices so we’re continually able to offer recommendations to refine manufacturing processes.
Risk 5: Complications related to cell administration and toxicities
How we address this:
We select sites and principal investigators with field experience in administering cell therapies. Familiarity with cell therapies and potential complications makes observing and managing adverse events more reliable.
Patients are made aware of potential complications such as cytokine release syndrome (CRS), neurotoxicity and infections through educational materials provided during informed consent. Additionally, to support trial sites, we can also provide guidance to monitor, report and manage possible side effects or health complications. Many protocols offer specific treatment algorithms for commonly observed side effects, along with criteria for administering standard medications to manage them.
Additionally, before the start of a clinical trial, our team ensures that clinical sites have the necessary supply of medications stocked in their pharmacy to immediately manage patient complications. If necessary, we also provide recommendations for alternative sources of medications so there are no shortages or delays.
Risk 6: Sustained participation in long-term follow-up
How we address this:
Due to the relative novelty of cell therapies, long-term follow-up processes may not necessarily have gold standard practices. However, through our experience, we’re able to observe strategies that boost patient compliance and can make recommendations to sponsors based on current or past best practices that have been successful.
Through tele-visits that provide virtual health monitoring or through our mobile clinical services, where nurses or physicians go to a patient’s home to monitor health and collect samples, we aim to minimize patient burden and costs associated with long-term follow-ups, thereby hoping to improve sustained participation. We’re also able to localize patient sample testing by tapping into our global network of clinical laboratories. Fortrea’s solution ensures the key data such as persistence of cells infused or delayed adverse events related to cell/gene therapy are collected during the long-term follow-up part of the trial.
Finally, to pave the path for future cell therapy programs, we’re continually engaged in dialogue with regulatory bodies to develop well-defined strategies that can support the success of extended long-term follow-ups.
Conclusion
At Fortrea, we value the perspectives of the different stakeholders involved in administering cell therapies, placing the patient experience at the highest priority. We understand how sponsors and clinical trial sites need to have de-risking measures in place so that patients don’t need to wait any longer to receive life-changing therapies. Having seen the cell therapy field evolve and having had the opportunity to play a significant role in its development by supporting all 6 FDA-approved CAR T-cell therapies, we’re able to identify, anticipate and even take preemptive steps to minimize risks.
Reach out to our cell and gene therapy team to start a conversation about how we can offer support to de-risk and accelerate your program.
About Fortrea
Fortrea is a leading global provider of clinical development and patient access solutions to the life sciences industry. We partner with emerging and large biopharmaceutical, biotechnology, medical device and diagnostic companies to drive healthcare innovation that accelerates life-changing therapies to patients. Fortrea provides phase I-IV clinical trial management, clinical pharmacology, consulting services, differentiated technology enabled trial solutions and post-approval services.
Fortrea’s solutions leverage three decades of experience spanning more than 20 therapeutic areas, a passion for scientific rigor, exceptional insights and a strong investigator site network. Our talented and diverse team working in more than 90 countries is scaled to deliver focused and agile solutions to customers globally.