
Digital therapeutics: The key to maximizing the potential of medicinal assets
Key takeaways:
- Digital therapeutics support increased access to safe and effective therapies, providing an untapped opportunity for biopharmaceutical companies to maximize the value of medicinal assets and drive differentiation
- When developed in combination with a medicinal asset, digital therapeutics enable significant market differentiation and significant benefits for stakeholders—from optimizing patient outcomes to supporting more efficient generation of compelling real-world data
- Integrating digital therapeutics early in the asset development process will allow for gathering of clinical data to drive a clearer value proposition that maximizes the potential of both products
- Early market entry is fundamental, and manufacturers must plan strategically to consider how digital therapeutics (DTx) are best incorporated into the launch and go-to-market strategy. Internal digital and data science expertise is critical to developing a united value proposition that aligns the benefits of the digital and medicinal product
This is a summary, taken from Fishawack Health’s new publication “Digital therapeutics: The key to maximizing the potential of medicinal assets.” Download it here.
It is estimated that the number of people using digital therapeutics will increase by 381% over the next four years.1
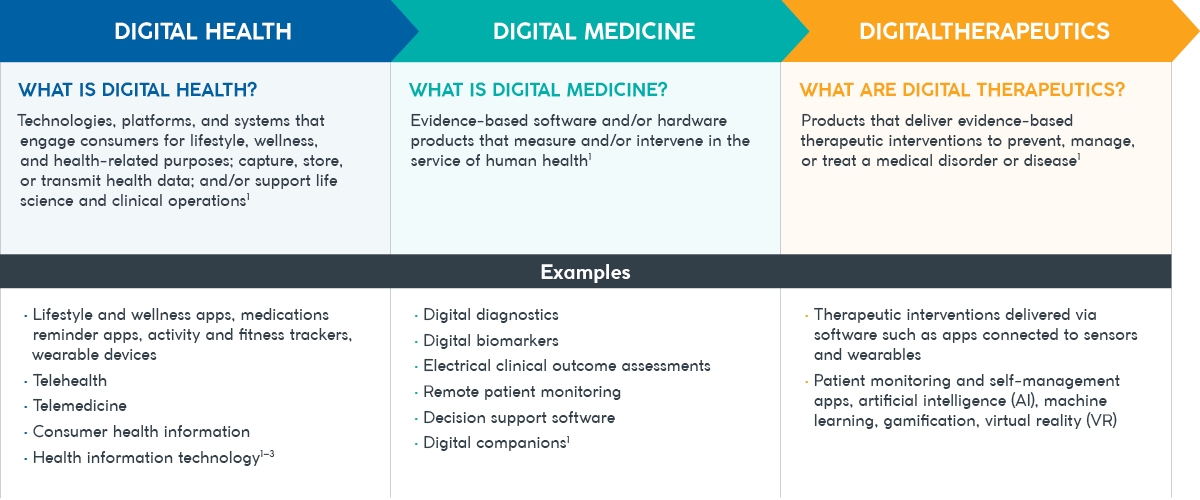
Digital therapeutics are a subcategory of digital medicine. They can be categorized into three subtypes depending on whether they are designed to prevent, manage, and/or treat a disease or condition.
The key difference between digital medicine and digital therapeutics is that digital medicines require clinical evidence alone, whereas digital therapeutics require both clinical evidence and real-world outcomes data. This means digital therapeutics are held to the same standards of evidence and regulatory requirements as conventional pharmacological treatments, requiring approval from regulatory bodies to support product claims of risk, efficacy, and intended use.2
Figure 1: The key differences between digital health, digital medicine, and digital therapeutics2
Proactively integrating digital therapeutics into your early global commercial asset strategy can increase the chances of a successful launch and commercialization, ensuring assets are positively differentiated from the competition and offer benefits beyond the drug for all stakeholders, including:
- Improved outcomes, access, and experience for patients
- Healthcare professionals can improve care for patients through additional monitoring, alongside leveraging applications that support patient adherence
- Scalable and cost-effective interventions for payers, including improved clinical and health economic outcomes, reduced cost of medical interventions, and the ability to collect real-world data
For biopharmaceutical manufacturers, the benefits are far reaching and include the ability to differentiate assets positively in crowded markets with multiple competing modes of action and where several generations of a product are on the market simultaneously.
Integrating a digital therapeutic into the asset strategy can also help drive efficient clinical trials and support effective evidence generation, providing more compelling data to regulators and payers to achieve favorable access.
However, the window of opportunity for biopharmaceutical companies to develop digital platforms that can be optimally combined with assets to fill unmet needs is closing. Manufacturers need to act quickly, before the tech giants shape this arena, to develop digital therapeutics that truly increase the value of products for all stakeholders.
Key considerations for biopharmaceutical companies to align on before entering the DTx market
Before entering the market, manufacturers must plan strategically with a future-focused lens to maximize the value of the asset and portfolio, ensuring a clear vision and business case to support the commercialization of the digital therapeutic, which includes the following considerations:
1. A united value proposition
Digital therapeutics and asset development should not be seen as separate but of the same overall value proposition and total product profile (ToPP). This mindset ensures truly synergistic development, overcoming barriers, and maximizing potential.
Embedding digital therapeutics into the overall downstream development process must complement existing processes without causing additional constraints. To achieve this, biopharmaceutical companies will need deep knowledge of the existing processes and systems, using design thinking and deeper insight methodologies to drive integration.
2. Early integration into research and development
Digital therapeutic development is not a quick process. To ensure maximum value for both patients and companies, it must be integrated early in the asset research and development process. This enables the gathering of evidence of clinically significant outcomes, which are integral to the digital therapy approval processes.
With the prediction that digital therapeutics markets will intensify due to both biopharmaceutical and tech companies entering this space, it is critical the narrative is shaped proactively, highlighting the importance of early market entry. Teams must consider how they iteratively test human-centric digital therapeutic solutions and scale promising aspects in the same way they would with a non-digital therapy.
3. Digital and data science expertise and mindset
Building the required capabilities and expertise within technology, data science, and analytics is critical and takes planning and timing. Bringing teams on this transformation journey will require new processes and structures. Understanding the needs of and connecting with a wide range of stakeholders (KOLs, PCPs, patients, caregivers, regulators, payers—and specific segments of each) is essential for ensuring a successful market uptake. It is imperative that your target stakeholders can see the significant value that your digital therapy can provide.
The needs of your stakeholders are key to creating a product that provides value to an area of unmet need for all stakeholder groups. Strong collaborations and partnerships will ensure that the overall ToPP provides a compelling offering.
4. Launch and market strategy
An altered launch strategy is required to ensure the launch of both the asset and digital therapeutic are firmly established and the targeted value is achieved within the real world, particularly as the market becomes more competitive and ambiguous.
Get the full analysis
This is a summary taken from Fishawack Health’s new publication “Digital therapeutics: The key to maximizing the potential of medicinal assets.”
Download the whitepaper to gain insights on:
- Digital therapeutics market growth
- Key therapy areas and applications
- Detailed benefits for stakeholders and advice on overcoming adoption hurdles
- Strategic recommendations for biopharmaceutical companies for developing a digital therapeutics strategy in combination with asset commercialization
References:
- Juniper Research. https://www.juniperresearch.com/researchstore/healthcare-government/digital-therapeutics-market-research-report; 2022 [accessed January 13, 2023].
- Digital Therapeutics Alliance. https://dtxalliance.org; [accessed January 12, 2023]
About the Authors
Kirsten Rennie, Senior Associate Consultant, Fishawack Health
Kirsten has experience in go-to-market strategy, early asset brand strategy, digital transformation, omnichannel engagement strategies, and tactical roll-out for global brands. She is passionate about improving patient outcomes globally through the adoption of innovations such as digital health and how these can be harnessed to increase patient access to life-saving therapeutics.
Hassan Choudhury, PhD, Early-Stage Strategy Lead, Fishawack Health
Hassan focuses on maximizing the potential of assets during early‑stage development through to launch and the implementation of go-to-market models. He is passionate about delivering improved outcomes to patients through the integration of deeper insights earlier and challenging norms in the pursuit of advancement for patients and healthcare systems.
About Fishawack Health
Established in 2001, Fishawack Health (FH) is a purposefully built commercialization partner for the biopharmaceutical, medical technology, and wellness industries. Our 1,400+ experts combine their knowledge and expertise across our core disciplines—Medical; Marketing; Policy; Value, Evidence, and Access; and Consulting—to create the connections that make better health happen. We partner with our clients to navigate the complex and rapidly changing healthcare ecosystem. Together, we realize the potential of strategies and solutions to bring innovation to the hands of those who can benefit from it.