Five Steps to Guide Successful Risk-Based Decentralized Clinical Trials
DCTs are not a brand new idea. For more than a decade, the drug and medical device development industry has been trying to answer the question: How do we bring the study to the patient? DCTs offered a potential answer, yet companies had been wary to adopt this previously relatively untried model.
Now, bringing trials to patients is no longer a nice-to-have, as more than half of the top 50 pharmaceutical companies have had to make protocol changes in their ongoing trials since the pandemic began and others have paused trials completely. As of May 20, one-third of sponsors were switching to virtual or decentralized models, according to the Tufts Center for the Study of Drug Development (CSDD).{1} And on September 2nd, FierceBiotech reported a new survey showing that two-third of healthcare experts plan to use virtual trials as a result of Covid-19 (Fierce Biotech). {2}
Long after the pandemic subsides, companies will recall these dark days and want to be prepared to minimize the risk of trials being disrupted again as well as provide more choices for engagement to sites and patients. Here are five key steps to help sponsors successfully decentralize in-flight trials and start new DCTs within a risk-based framework.
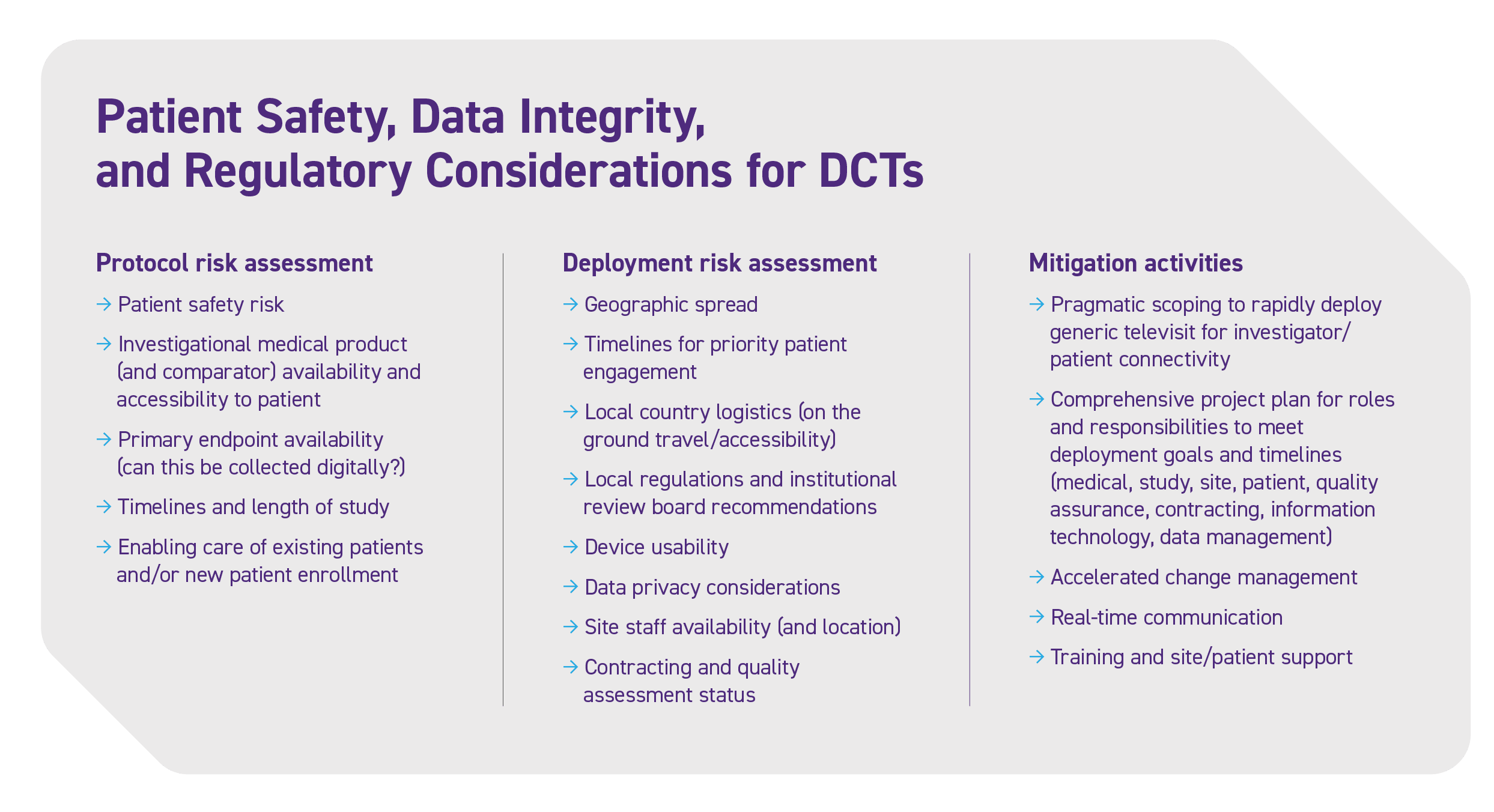
- Document a specific decentralized study design and implementation plan, including all patient safety, data integrity, and regulatory considerations (see Figure 1).
- Determine the wearables and devices that are needed, what tools are already being used in the study ecosystem (i.e., interactive response technology, electronic data capture, central labs, etc.), and other types of support that would be needed (i.e., home health nursing, local labs, etc.).
- Evaluate how decentralized data will be reviewed and monitored to ensure quality and integrity. For example, define which data are collected for remote patient safety oversight monitoring and which for study endpoint analysis.
- Equip all sites for success in a decentralized model, considering how it will impact efficiency and daily operations. Map out the options that would be available for each site, including those that are acceptable within a site’s infrastructure already, to leverage different technologies or remote support teams. Additionally, consider what support and training will be needed to enable the sites to engage effectively in DCTs.
- Enhance the patient/physician relationship in a virtual world by providing options such that study teams, investigators, and patients have choices about how they can participate in a study. It’s important to recognize that one size does not fit all, so flexibility is crucial to allow for physician and patient choice in order for DCTs to be efficient and effective for both parties.

All of the news, delivered with full-text to your inbox. For professionals discovering, developing, and marketing biopharmaceutical drugs.
DCTs are Proving Themselves as a Successful Invention That’s Here to Stay
From the largest to the emerging, life sciences companies are embracing DCTs, and so are their partners and patients. A recent Site Landscape Survey by the Society for Clinical Research Sites revealed that more than half of sites reported they would do “whatever is required” to conduct a virtual or decentralized trial, while 75% of patients say that collecting all study data from their own home is appealing.{3}
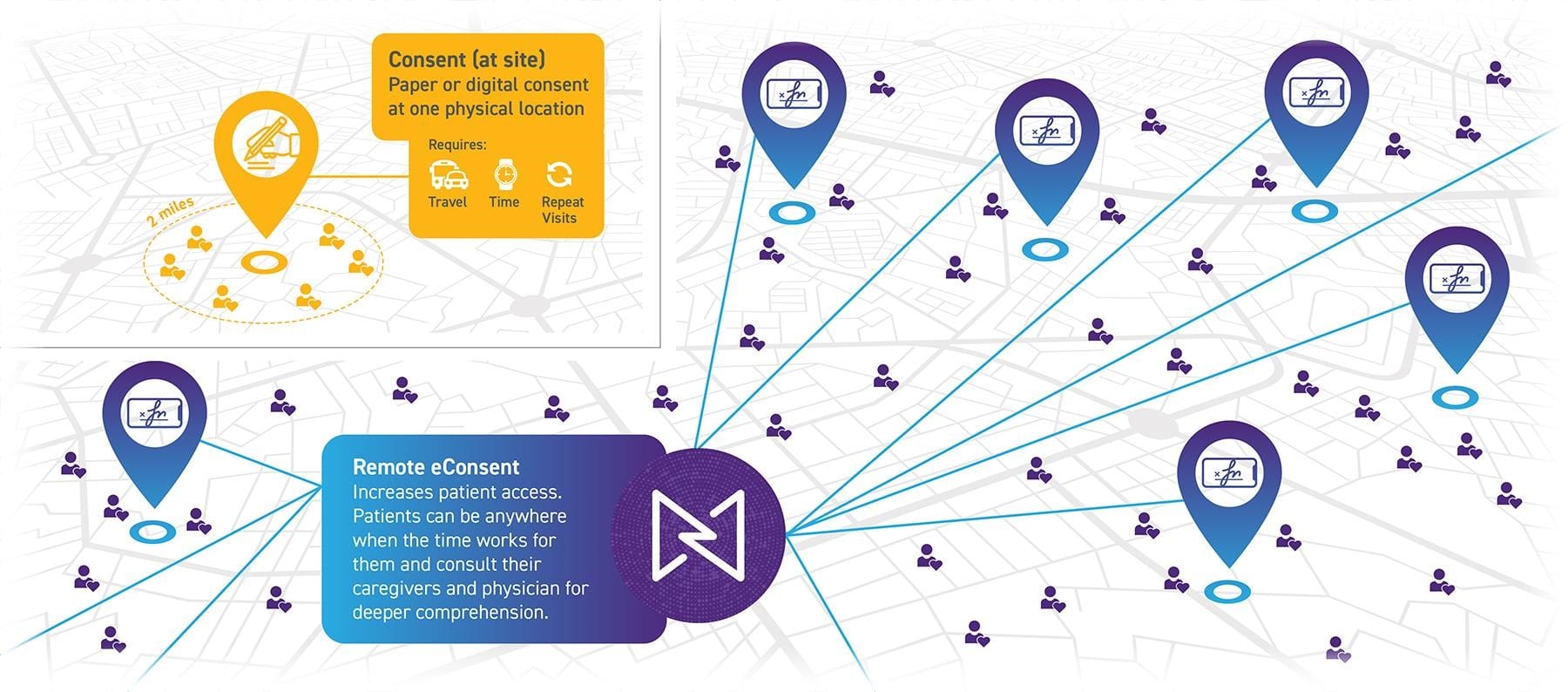
Decentralized Trial Technologies like remote recruiting and eConsent can dramatically expand patient access.
Click on the image to see the full-sized version
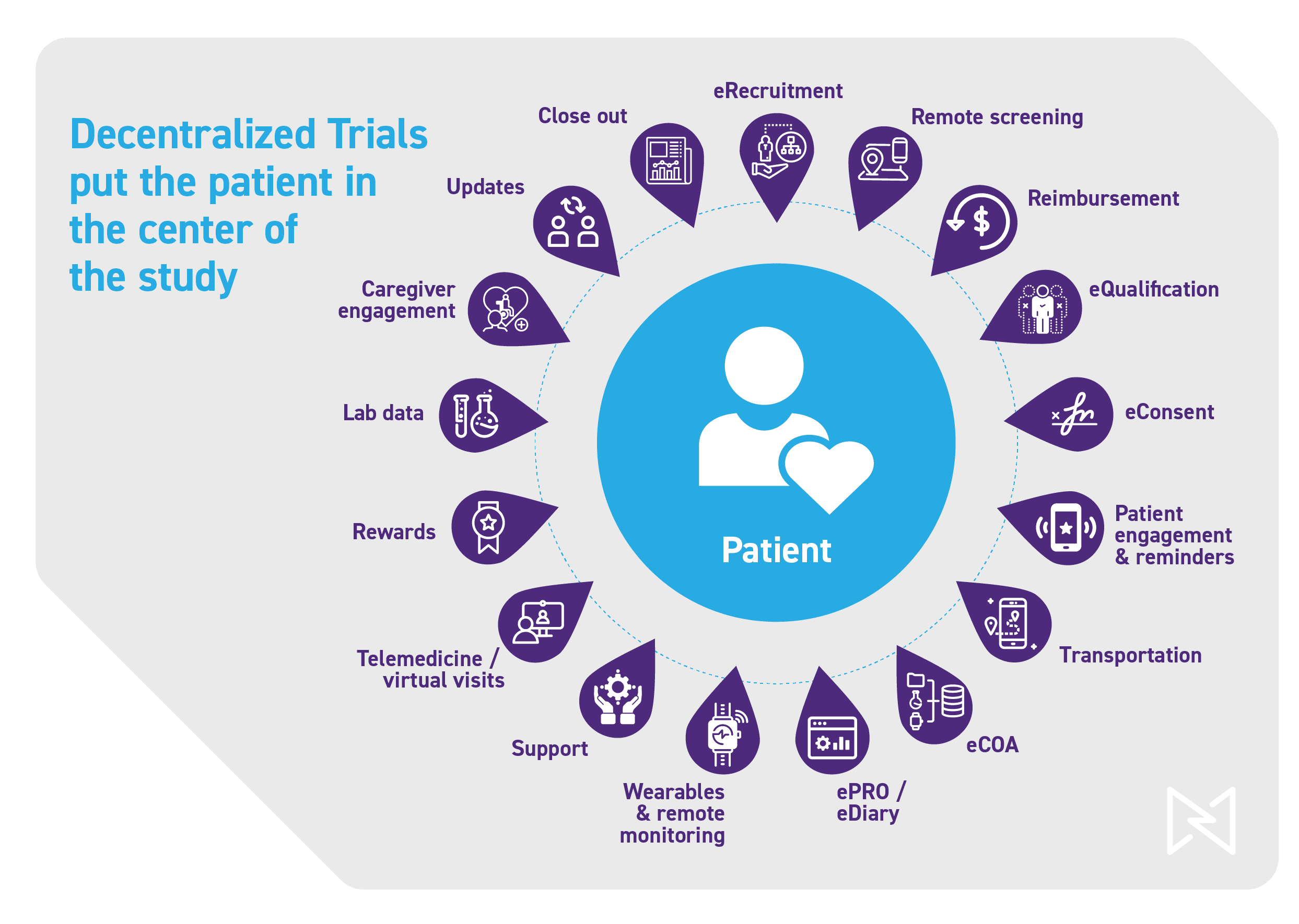
Clinical research, wherever it occurs, will always require the expertise and experience of qualified teams that embrace a risk-based approach. By implementing a “patient first” DCT, organizations can shatter the traditional study paradigm and finally achieve sought-after improvements in patient accessibility, unification of data, and data availability, culminating in faster, more effective trials.
We have the ability to engage with our patients, learn about their lives and priorities, and apply these insights to optimize the study design and mitigate upfront risks. For example, by employing technology to conduct pre-recruitment assessment data collection, companies can understand how people live so that protocols can be customized—bringing a patient-focused experience to clinical research.
While the COVID-19 pandemic has been a catalyst in forcing new clinical trial execution models to go mainstream, they will be part of a mix of options in the long term. The advantages are powerful and the results are the proof—DCTs are here to stay.
(1) CenterWatch. 2020. Pandemic Unleashes Momentous Change in Clinical Research. See full resource here.
(2) Fierce Biotech. 2020. Two Thirds of Healthcare Experts plot virtual trial use as a result of Covid-19: Report. See full resource here.
(3) Center for Information and Study on Clinical Research Participation (CISCRP). 2019. 2019 Perceptions and Insights Study: Patient Experience Report. See full resource here.