
Forge Biologics’ cGMP Compliant and Commercially Viable Bespoke Affinity Chromatography Platform
Forge Biologics has developed a bespoke affinity chromatography platform approach that factors in unique vector combinations to streamline development timelines and assist our clients in efficiently entering the clinic. By leveraging our experience with natural and novel serotypes and transgene conformations, we are able to accelerate affinity chromatography development by nearly 3-fold. Many downstream purification models are serotype-dependent, demanding unique and time-consuming development strategies for each AAV gene therapy product1. With the increasing demand to propel AAV gene therapies to market, platform purification methods that support commercial-scale manufacturing of high-quality vectors with excellent safety and efficacy profiles are essential.
Forge has established a bespoke purification process applicable across a variety of AAV vectors. When affinity chromatography process development or targeted optimization is required for a specific vector, the Forge team approaches this in a calculated manner making data-driven decisions based on our platform development studies. Rather than starting from scratch and evaluating combinations of resin, binding capacity, and buffer compositions, only minor adjustments are needed, for example, a change in the pH of the elution buffer.
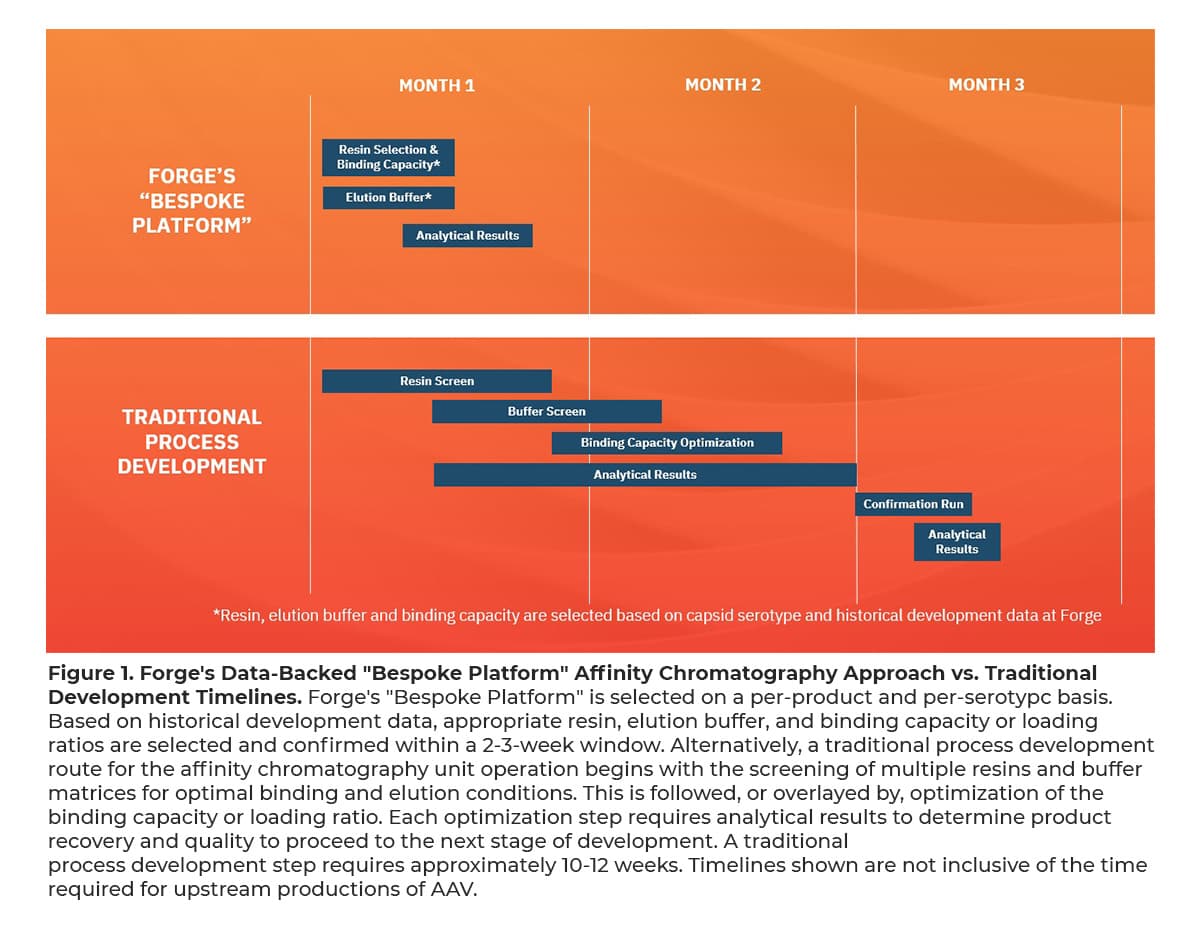 With three AAV gene therapies on the market and several more anticipated regulatory decisions this year, scalable, commercial-ready downstream processes are needed to meet current and future manufacturing demands. Furthermore, with the industry rapidly advancing, rare and high prevalence disease indications, capacity, as well as scalability of processes are required in tandem. Forge has built a state-of-the-art manufacturing facility committed to 200,000L+ per year that uses single-use affinity chromatography flow paths to enhance reproducibility between productions and mitigate risks associated with cross-product contamination.
With three AAV gene therapies on the market and several more anticipated regulatory decisions this year, scalable, commercial-ready downstream processes are needed to meet current and future manufacturing demands. Furthermore, with the industry rapidly advancing, rare and high prevalence disease indications, capacity, as well as scalability of processes are required in tandem. Forge has built a state-of-the-art manufacturing facility committed to 200,000L+ per year that uses single-use affinity chromatography flow paths to enhance reproducibility between productions and mitigate risks associated with cross-product contamination.
Affinity Chromatography: A Commercially Viable Platform Approach to a Powerful Downstream Purification Technique
Affinity chromatography purification methods provide high selectivity, resolution, and capacity by exploiting a protein’s biological structure to purify it from solution. Affinity chromatography is the first step in Forge’s platform downstream AAV purification process and is comprised of an affinity resin stationary phase and mobile phases promoting capture or elution of the AAV product. Commercially available affinity resins, such as Cytiva AVB, and POROS™ CaptureSelect™ AAV9, AAV8, and AAVX, remain reliable assets to the AAV manufacturing field and are routinely screened and used at Forge during all stages of development.
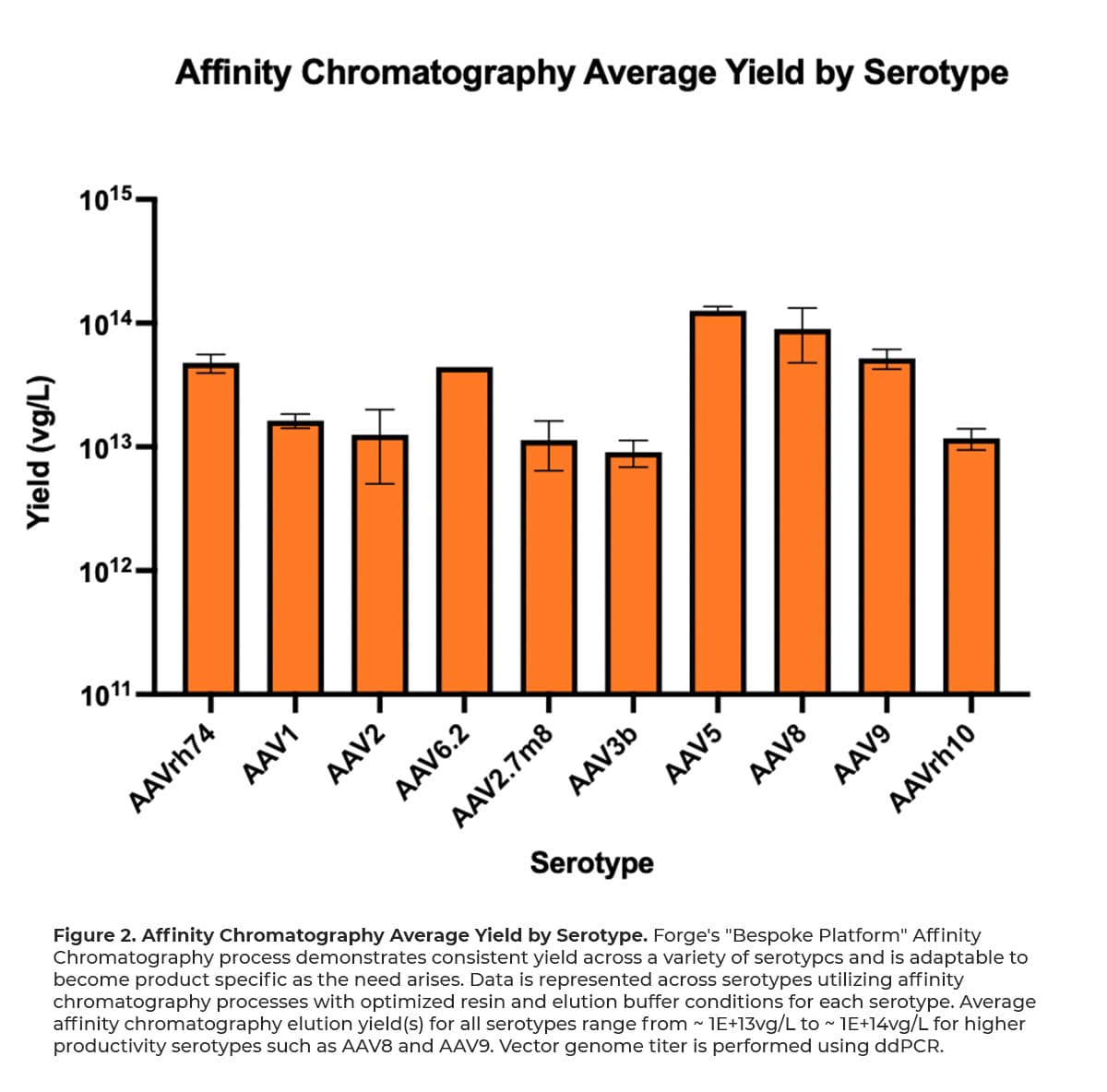
After initial capture of AAV, the dissociation and recovery of viral genomes from chromatography resins involves a disruption in binding interactions of the AAV from the immobilized ligand by altering the buffer composition. Forge’s universal buffer matrices for equilibration, washing, and eluting AAV particles from affinity resins were consciously designed for broad applicability to a variety of capsid serotypes. Figure 2 below highlights the proficiency of Forge’s affinity platform across a variety of serotypes.
 Process Challenges and Solutions for Scaling Affinity Chromatographic Purification of AAV Vectors
Process Challenges and Solutions for Scaling Affinity Chromatographic Purification of AAV Vectors
Purification of AAV at scale remains challenging and costly due to the handling of large working volumes, the difficulty in removing impurities, and the complexities in recovering vector genomes. Top considerations during the scale up of affinity processes include, but are not limited to, 1) AAV stability, 2) resin binding capacity, and 3) residence time.
- AAV Stability: Processing time is a key consideration when moving directly into affinity chromatography from clarified harvest, as extended processing times can impact product stability. While there are methods to combat the extended processing times of large-scale affinity chromatography (such as an ultrafiltration / diafiltration (UF / DF) step post-clarification), additional processing steps introduce risk for vector loss and increase the cost of goods per batch.
- Resin Binding Capacity: The binding capacity of an affinity chromatography resin dictates the concentration range of viral genomes that can be effectively loaded onto the resin. Universal and high-specificity resins advertise a binding capacity of 1012 – 1014 vg/mL resin. While the upper limit of the binding capacity is cost-effective by requiring less resin, the lower limit of the binding capacity can provide processing time efficiency.
- Residence Time: The exposure time of AAV to the resin in the affinity column (i.e., residence time) also impacts the total processing time of the affinity unit operation.
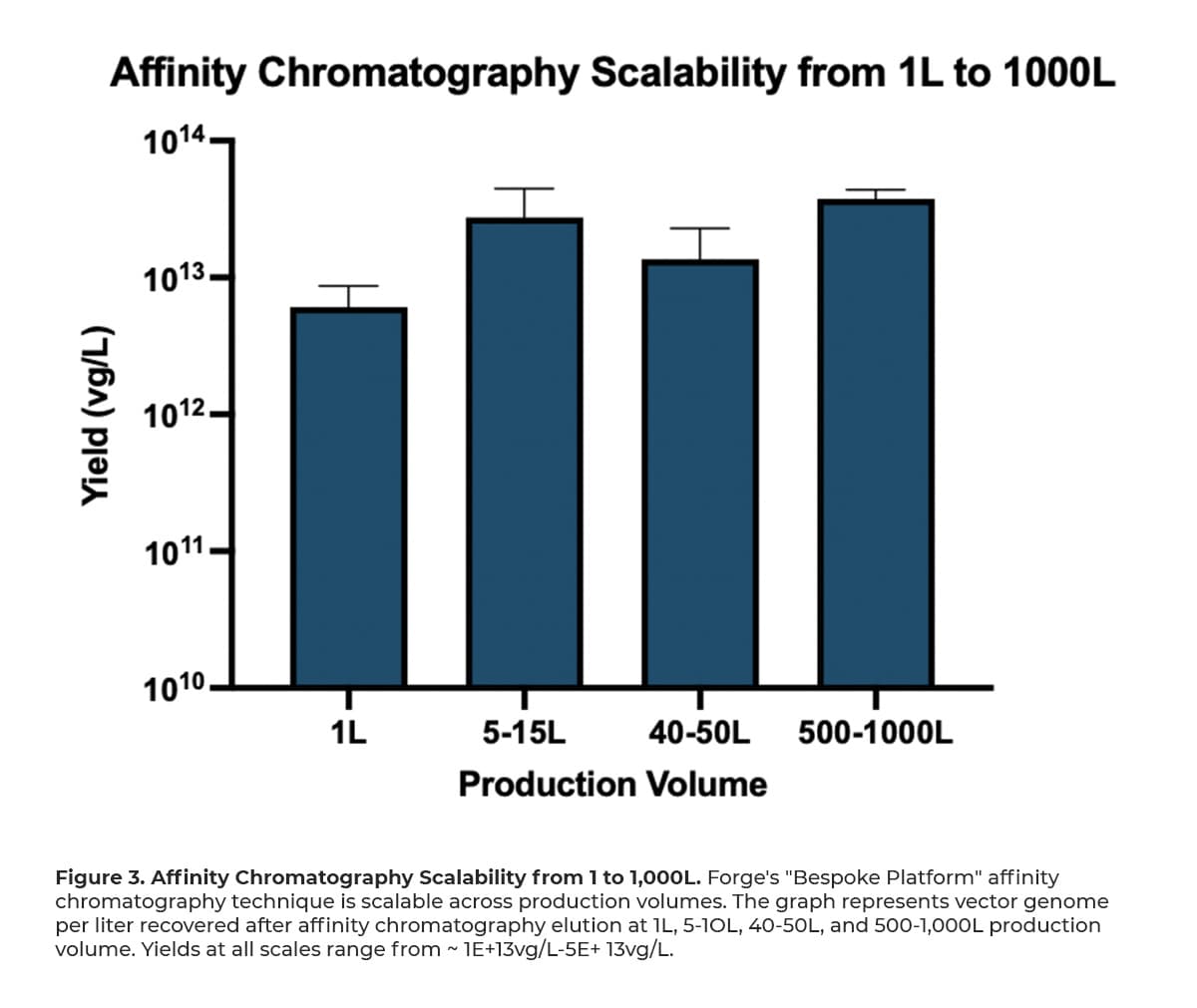
Forge’s approach to a scalable affinity chromatography step maintains a manageable and consistent total processing time. As our data supports, the increase in residence time with increasing scale does not negatively impact the affinity chromatography process, as seen in Figure 3, and is a parameter that is optimized when linear velocity decreases. Establishing large-scale chromatography processing systems and protocols for cGMP manufacturing that are comparable to bench-scale models is a familiar obstacle that Forge considers early during process development. Forge’s affinity chromatography platform leverages the Repligen ARTeSYN and Cytiva ÄKTA Ready™ 450 single-use chromatography systems and has demonstrated process efficiency and increased recoveries at scale.
 Forge Biologics increases safety and efficacy by providing a commercial-ready process through:
Forge Biologics increases safety and efficacy by providing a commercial-ready process through:
- The use of single-use flow paths, recipe-driven, and automated affinity chromatography methods to mitigate risks associated with cross-product contamination, as well as to enhance reproducibility between productions
- Process continuity and consistent processing time across scales
- Increased recovery and impurity removal using the 21 CFR part 11 compliant Repligen ARTeSYN and Cytiva ÄKTA Ready™
Increased Product Quality and Downstream Success
A productive and consistent affinity chromatography method is a critical processing step for the success of further downstream purification. Host cell impurities, residuals, and “empty” capsids, which lack the gene of interest and therefore do not provide therapeutic benefit2, are byproducts of AAV manufacturing that must be removed or reduced during downstream purification. While affinity chromatography is unable to distinguish and remove empty capsids1, its ability to remove and reduce process impurities aids in the efficiency of the subsequent polishing step (i.e., CsCl ultracentrifugation gradient or anion exchange chromatography).
Impurity removal during affinity chromatography is reliant on developing effective wash step conditions. The goal of the wash step is to remove unbound particles and other proteins that bind nonspecifically to the affinity column. A few considerations for developing a wash step for affinity chromatography include optimal buffer conditions, flow orientation, and wash column volumes. Salt composition and concentration must be carefully balanced in the wash buffer to avoid premature vector elution while promoting efficient impurity removal. Flow orientation can also play an impactful role in removing nonspecific proteins which become more concentrated at the column entrance and less concentrated at the column exit. Utilizing up- and down-flow functions of your chromatography system during the wash step prevents nonspecific proteins from rebinding throughout the column and instead releases them from the resin into the wash fraction. The amount of wash buffer that is passed over the resin in the two flow orientations (up-flow & down-flow) can drastically affect the purity profile of the final AAV product.
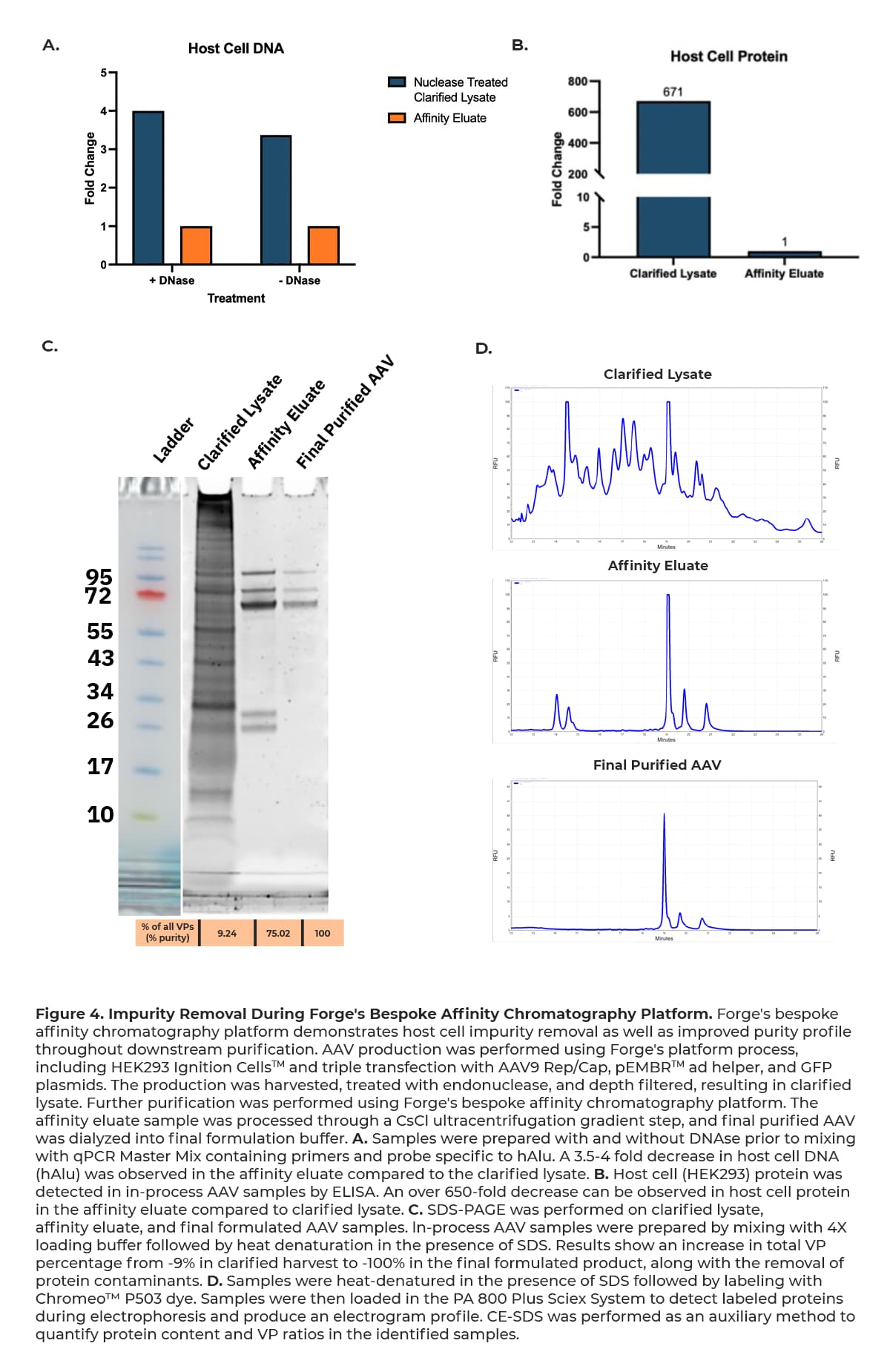
Forge’s bespoke platform demonstrates noteworthy removal of host cell DNA, host cell protein, and total protein impurities at the affinity chromatography step as shown in Figure 4 below, providing optimal material for further successful downstream processing.
 Conclusion
Conclusion
The AAV gene therapy industry is successfully tackling multiple obstacles for establishing scalable, commercial-ready downstream purification processes. Timelines for affinity chromatography process improvements can be reduced without negatively impacting product quality by approaching development from a data-backed starting point supported with process development groundwork completed by Forge on behalf of clients. Forge has overcome affinity chromatography processing challenges while maintaining or increasing recoveries when moving from benchtop to GMP scale systems. This demonstration of scalability utilizing single-use technologies for the purification of clinical material and Forge’s recent facility expansion are essential milestones in establishing and providing clients with a commercially viable process at 1,000L and greater.
References
- Universal Method for the Purification of Recombinant AAV Vectors of Differing Serotypes Shelley A. Nass,1 Maryellen A. Mattingly,1 Denise A. Woodcock,1 Brenda L. Burnham,1 Jeffrey A. Ardinger,1Shayla E. Osmond,1 Amy M. Frederick,1 Abraham Scaria,1 Seng H. Cheng,1 and Catherine R. O’Riordan1,∗https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5767896/#bib10
- AAV Empty Capsids: For Better or for Worse? J Fraser Wright1 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3978789/
Co-Author: Brianna Barrett, Ph.D., Associate Director, Technical Sales & Scientific Advisory, Forge Biologics
Contributors: Bryant Yung, Ph.D., Senior Scientist, Process Development, Forge Biologics and Mikhail Gavrilin, Ph.D., Associate Director, Analytical Development, Forge Biologics