Fueling More Breakthroughs: Manufacturing Innovation to Expand Gene Therapy’s Reach
Breakthroughs are on the rise in the gene therapy industry after decades of manufacturing challenges. Genetic therapies offer the potential to treat patients with some of the most complex and debilitating diseases. The industry has responded to these promising therapies with increased capacity for manufacturing and development and an ever-growing body of highly trained subject matter experts. However, technical hurdles—particularly in the commonly used HEK293 production system such as limited scalability, inconsistent or low yields, and time-consuming process development pathways—still pose challenges for developers trying to enter the clinic or eventually offer a commercially available product. As more gene therapy programs advance past early clinical stages and new programs begin targeting prevalent indications, the pressure to scale effectively and efficiently to meet this demand has intensified.
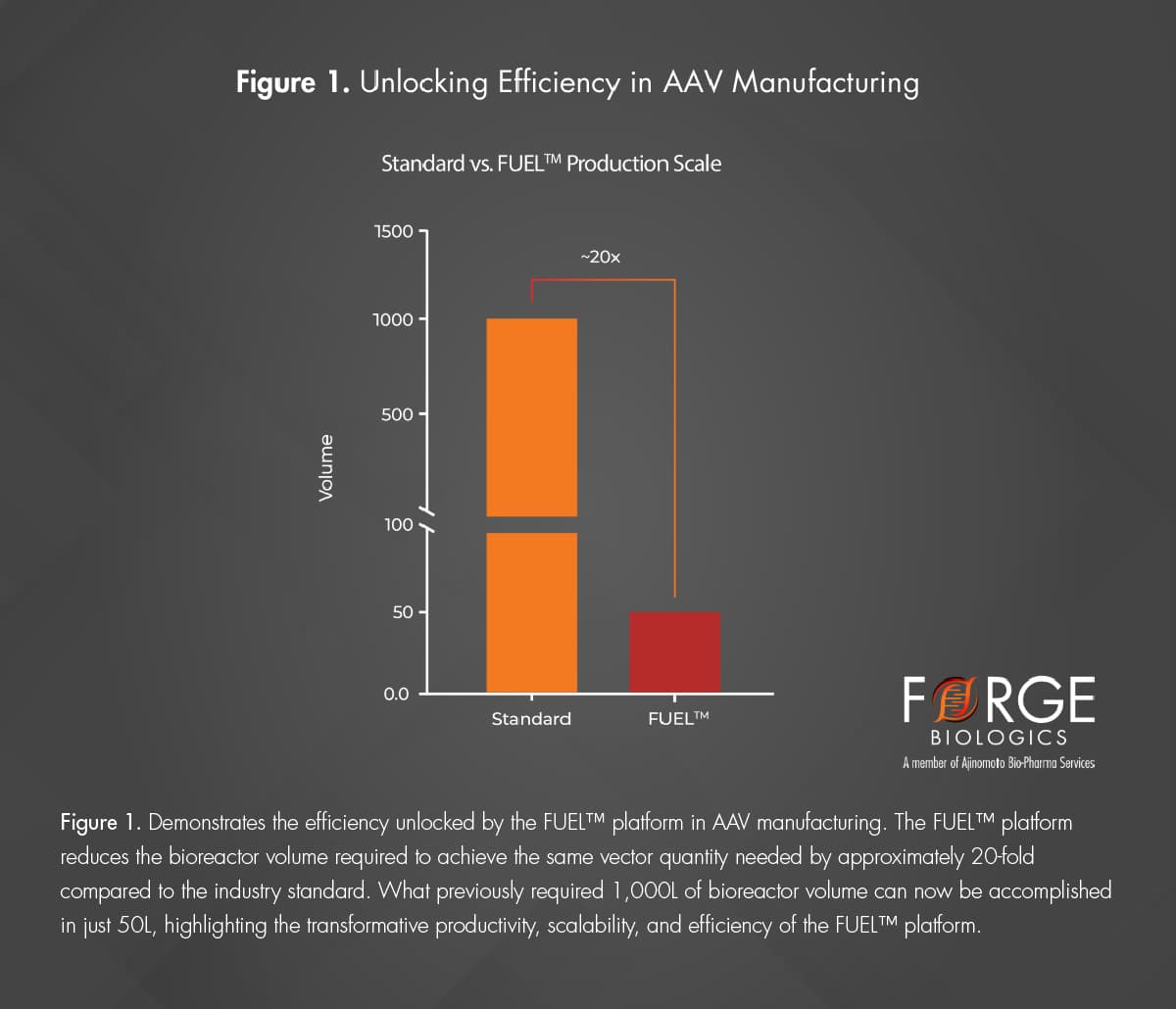
Despite advancements in gene therapy, accessibility remains limited by the need for higher production volumes to meet demand. Scaling up with larger bioreactors can increase production capacity but can also potentially raise costs of goods sold (COGS) and fails to address the critical efficiencies required to make these therapies broadly accessible. Overcoming manufacturing efficiency barriers is critical to ensuring gene therapies fulfill their potential to advance more programs and reach broader patient populations. Innovative manufacturing approaches like Forge’s FUEL™ platform aim to overcome these challenges by enhancing productivity and scalability. The FUEL™ platform reduces the total production volume needed to meet program requirements, as illustrated in Figure 1, making critical progress toward more accessible manufacturing.
Prioritizing Innovation and Product Specific Development to Unlock Efficiency in AAV Manufacturing
Forge is not only strategically scaling up and out to tackle the needed capacity but has also invested extensive time and resources into its foundational platform technologies with manufacturing research and development (R&D) to address manufacturing efficiency barriers such as COGS. The new FUEL™ platform incorporates molecular improvements in Ad helper and rep/cap plasmids into Forge’s proven and adaptable manufacturing process. The FUEL™ platform can also accommodate product specific process optimizations or alternative enhancers and additives as they become available in the field. Forge’s FUEL™ platform provides an advanced starting point to accelerate gene therapy development and lays the groundwork for program success.
The Technology Advancements of FUEL™
Optimized pEMBR 2.0™ Ad Helper Plasmid
The adenoviral (Ad) elements that are required for the successful production of recombinant adeno-associated virus (AAV) have been well established for 27 years1. These critical Ad components facilitate efficient replication and packaging of AAV vectors in mammalian cells. However, Forge has gone beyond conventional understanding, being the first in identifying another critical region encoding the Ad L4 22/33K proteins, which expands the industry’s understanding of AAV manufacturing requirements. Experiments conducted by Forge in 2023 confirmed that Ad helper plasmid variants lacking L4 22K protein encoding sequence were unable to support AAV production. Yet, by supplying the L4 22K gene, AAV yields were fully restored. Additionally, Forge’s novel studies showed that the L4 33K protein, when expressed synergistically, could further boost AAV production2. These findings have since been verified by Doshi, Jiten et al. and Johari, et al.3,4.
Building on these findings, Forge proceeded to refine and optimize its original pEMBR™ Ad helper plasmid. By removing extraneous Ad genes, Forge created a streamlined series of reduced size pEMBR 2.0™ helper plasmids (from 7 to 9 kb) that could enhance the AAV manufacturing process. The final pEMBR 2.0™ candidate was tested in small, mid-sized, and large-scale productions across various AAV serotypes and vector transgenes, showing exceptional performance in both single-stranded and self-complementary AAV production. The strategic modification of key genetic elements in the smaller pEMBR 2.0™ Ad helper plasmid resulted in significant increases in AAV yield, while potentially improving the overall safety of the design. pEMBR 2.0™ demonstrates a substantial improvement in the efficiency, manufacturability, and safety of AAV production.
Modified Rep/Cap Plasmids
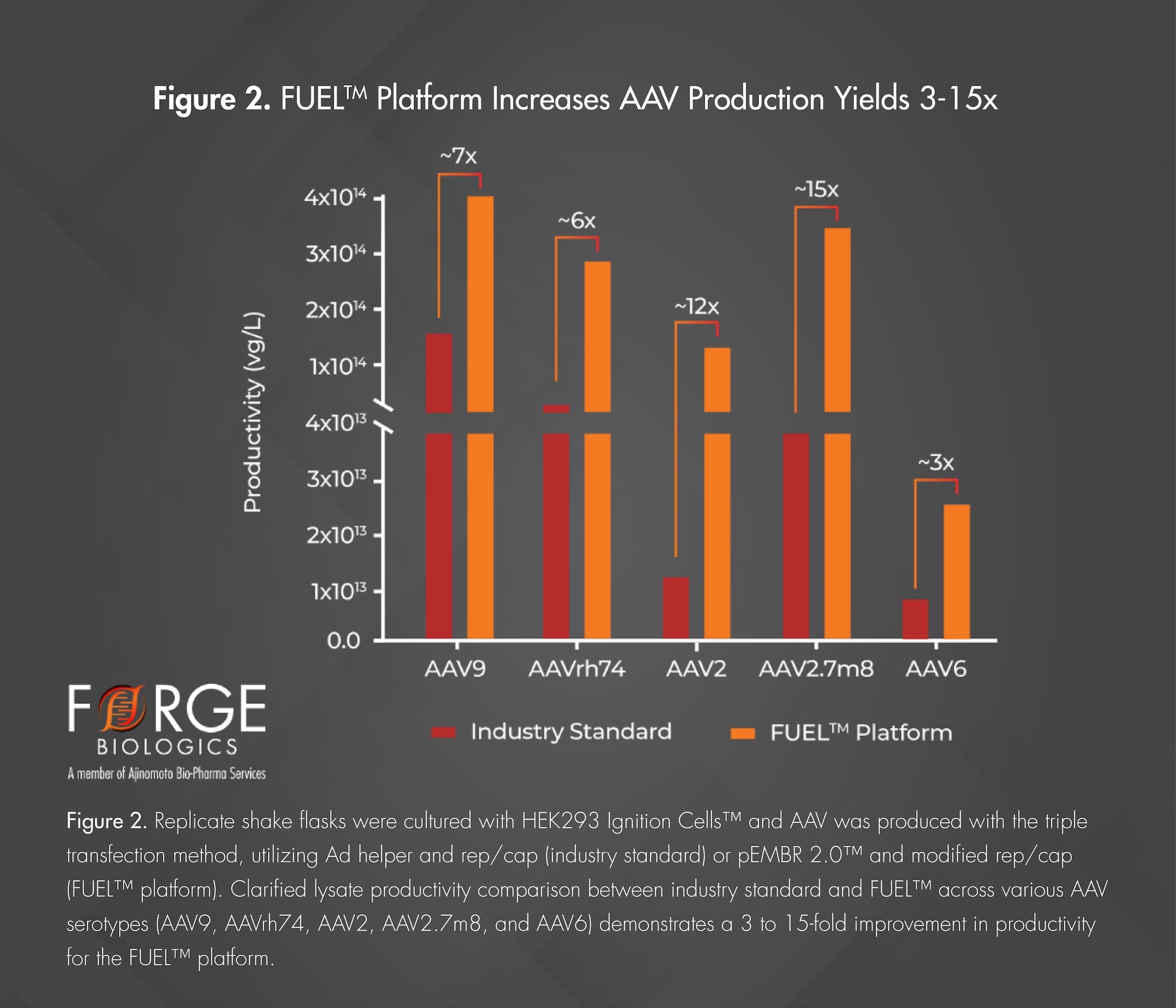
Forge has also made molecular improvements in traditional rep/cap plasmid design, employing techniques such as gene shuffling, codon sequence alterations, and site-directed modifications. These improvements have led to substantial increases in AAV yields across multiple serotypes, including the commonly utilized AAV2, AAV6, AAV9, AAVrh10, and AAVrh74. In addition to the yield increase seen with modified rep/caps alone, when combined with pEMBR 2.0™, an additional 3 to 15-fold increase in AAV yield has been observed, depending on the serotype, as seen in Figure 2. This advancement within the molecular foundation of AAV production marks an essential boost to AAV manufacturing efficiency, making high yields attainable in manageable volumes. While both rare and prevalent indications stand to benefit from a substantial reduction in manufacturing COGS, prevalent indications will also benefit from a movement towards clinical and commercial manufacturing within a manageable footprint.
Ignition Cells™
Forge’s proprietary suspension HEK293 Ignition Cells™ are a foundational technology available for all clients to use as part of the new FUEL™ platform. The cell line has a well-documented history and has been optimized for robust transient transfection, enabling streamlined production of AAV vectors at scale. Forge’s GMP Master Cell Bank (MCB) is fully qualified and delivers consistent, high-quality AAV production performance.
Proven Manufacturing Processes Honed by a Team of AAV Experts
In the fast-paced field of gene therapy, the race towards patient access to treatment has led many developers to rely on traditional platform processes and their ability to manufacture therapies reliably, at scale, and with consistent quality. As the field reaches beyond ‘proof of concept’ and equips for long-term commercial viability, technical advancements in manufacturing are key. The FUEL™ platform is intentionally designed to address some longstanding challenges in the HEK293 production system for AAV manufacturing, offering the scalability, flexibility, and efficiency needed to bring these transformative therapies to their next phase of growth.
By iterating upon its platform processes and successfully completing nearly 500 runs across a wide range of serotypes—including novel capsids—Forge has applied substantial insights to the development of the FUEL™ platform. Standard upstream production and downstream purification parameters remain largely unchanged, but for adjustments to scale due to the benefits of the improved FUEL™ platform.
Platforms Are Not a One-Size-Fits-All Model: Optimization Work Packages to Meet Individual Program Needs
The FUEL™ platform offers a robust baseline manufacturing process, along with a suite of optional optimization packages designed to refine and customize the process to meet specific program needs. These packages target improvements in yield, recovery, and product purity.
Transfection Optimization Package:
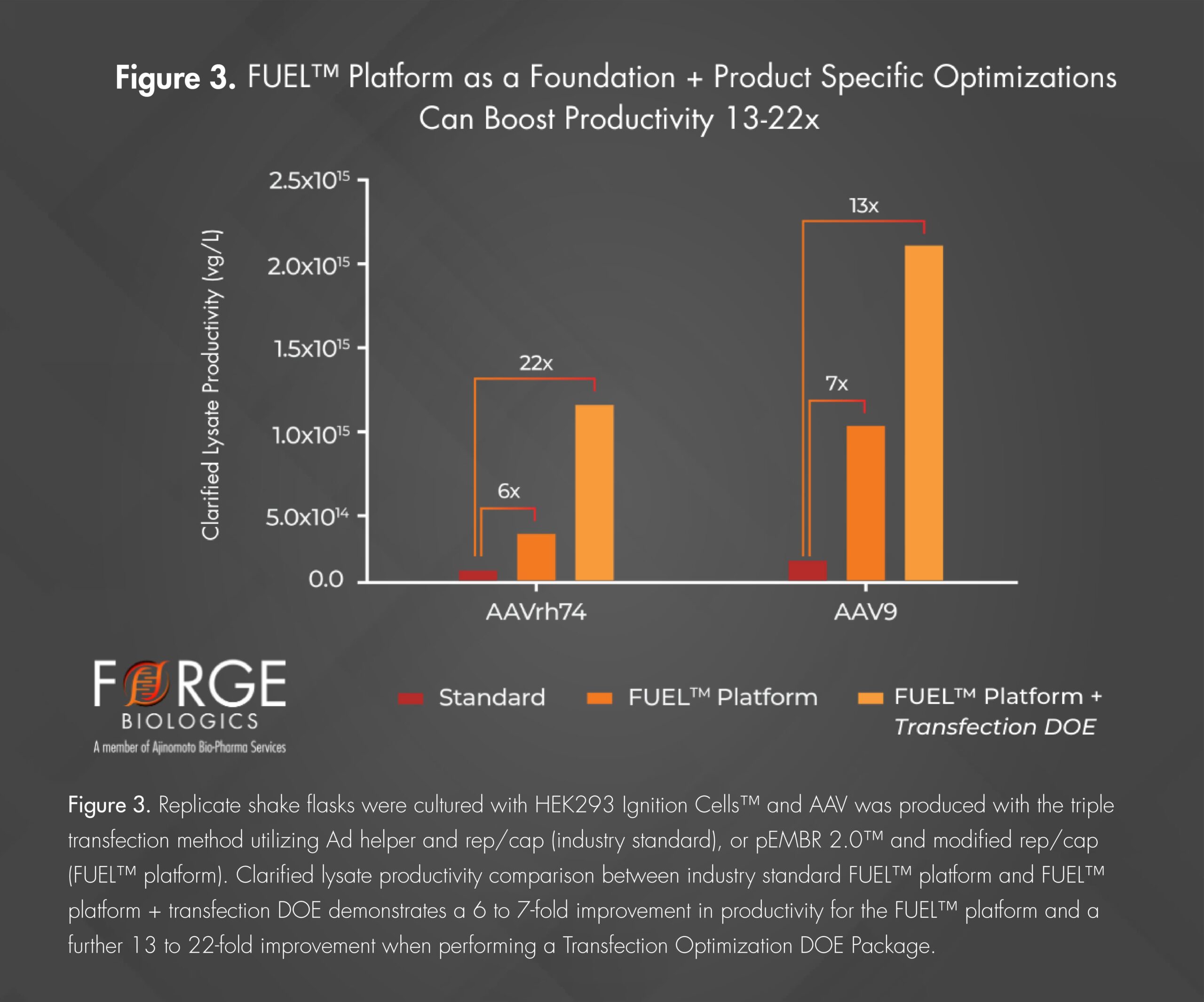
Gene therapy manufacturers have increasingly focused on maximizing upstream productivity as a key strategy to enhance total purified production yield. The Transfection Optimization Package uses a Design of Experiment (DOE) approach to enhance the baseline performance of the FUEL™ platform by creating a predictive model and screening potential transfection conditions aimed at boosting AAV production yields such as DNA per cell, or plasmid ratios, as well as screening of reagents that may act as enhancers of the AAV production process. This optimization package in combination with the FUEL™ platform process has demonstrated productivity increases from 1.59E+14 vg/L to 2.00E+15 vg/L for AAV9, as seen in Figure 3.
Downstream Optimization Packages:
There are also product-specific downstream processing optimization work packages that offer tailored solutions to enhance both purity and recovery rates, with a focus on developing commercially viable processes.
Options for optimization of downstream processes include:
- Affinity chromatography optimization: Forge’s bespoke affinity chromatography platform serves as both a versatile framework and a tool for implementing further product-specific process improvements. This work package enables studies to assess binding capacity and optimize elution conditions, supporting scalable and cost-effective process development.
- Cesium chloride gradient optimization: CsCl centrifugation studies can be performed to assess load capacity for scalability, while anion exchange chromatography (AEX) studies are conducted to optimize product-specific protocols for large-scale purification.
- Tangential flow filtration (TFF) optimization: TFF studies may be performed to increase the purity of the final product, ensuring products meet dosing requirements and are prepared for clinical applications.
By fine-tuning both upstream and downstream processes, these work packages streamline manufacturing workflows, reduce costs, and help with faster time-to-market for critical therapies.
Preparing Your Genetic Therapy for Commercial Longevity
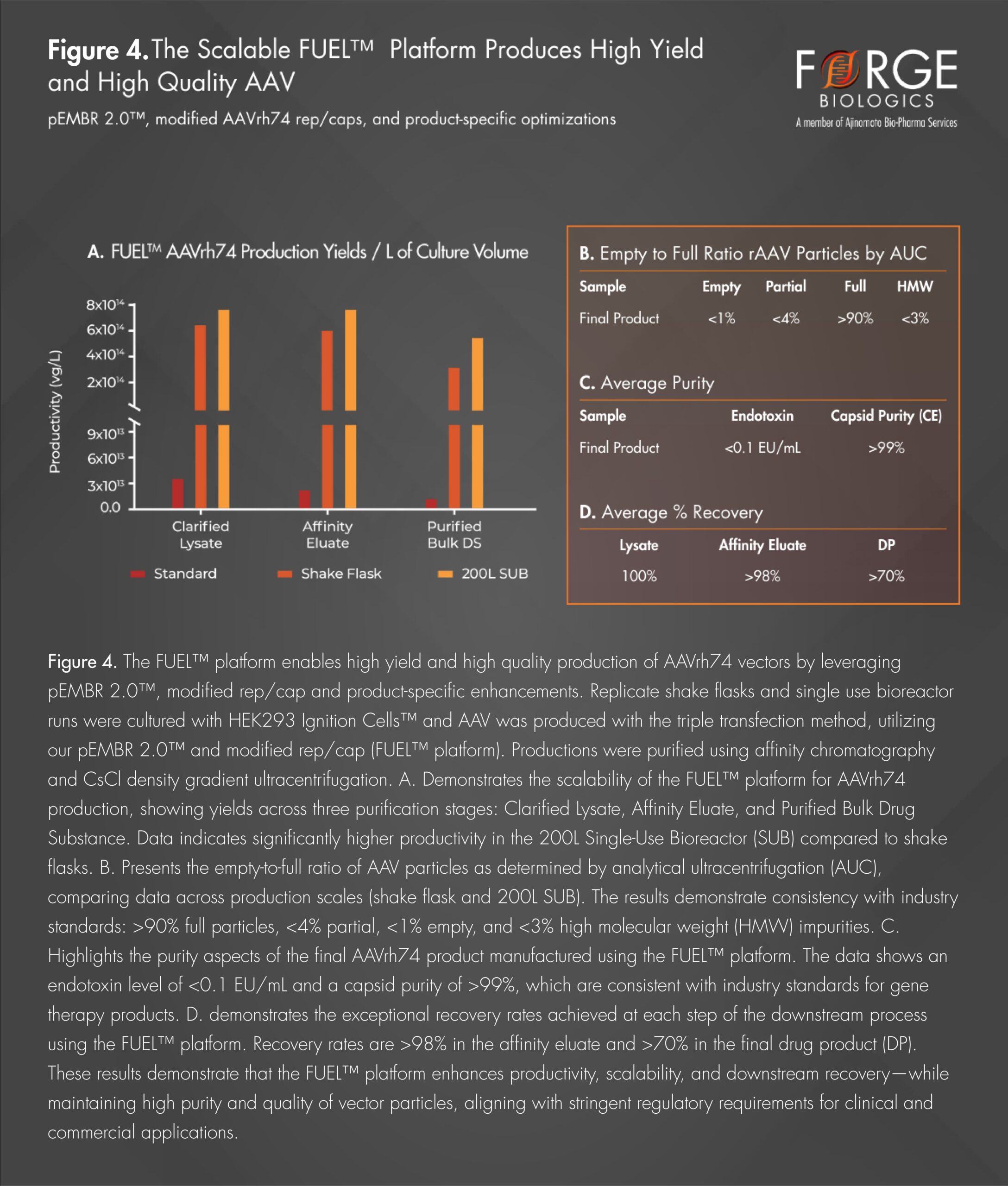
The modified rep/cap and pEMBR 2.0™ technologies within Forge’s FUEL™ platform have demonstrated the powerful ability to exceed industry standards for AAV production yields, while maintaining safety and quality. When combined with product-specific optimization packages, the platform demonstrates enhanced productivity, further supported by its scalability from a 1L shake flask to a 200L single-use bioreactor, ensuring seamless transition across development stages as seen in Figure 4. The result for therapeutic developers is a manufacturing platform that offers increased efficiencies while maintaining high product quality and safety. These productivity increases will be even more critical as developers move from clinical production into commercial manufacturing where the efficiencies gained at scale can help address the high COGS barriers that have the potential to limit a program’s commercial viability. With these increased efficiencies, Forge is uniquely positioned to help deliver high quality therapies to gene therapy developers’ patients worldwide, making our platform not only a foundation for unleashing excellence but also a catalyst for making gene therapies more accessible long term.
Learn more about how Forge can help fuel your gene therapy here.
References
- Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman GJ, Iwaki Y, Colosi P. (1998). Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998 Jul;5(7):938-45. doi: 10.1038/sj.gt.3300680. PMID: 9813665
- Adsero, A., Chestnut, B., Shahnejat-Bushehri, S., Sasnoor, L., McMurphy, T., Swenor, M., Pasquino, R., Pradhan, A., Hernandez, V., Padegimas, L., & Dismuke, D. (2023). A novel role for the adenovirus L4 region 22K and 33K proteins in Adeno-Associated virus production. Human Gene Therapy, 35(1–2), 59–69. https://doi.org/10.1089/hum.2023.146
- Doshi, Jiten et al. (2024). E2A, VA RNA I, and L4-22k adenoviral helper genes are sufficient for AAV production in HEK293 cells. Molecular Therapy Methods & Clinical Development, Volume 32, Issue 4, 12 December 2024, 101376
- Johari, Y. B., Pohle, T. H., Whitehead, J., Scarrott, J. M., Liu, P., Mayer, A., & James, D. C. (2024). Molecular design of controllable recombinant adeno-associated virus (AAV) expression systems for enhanced vector production. Biotechnology Journal, 19, e2300685. https://doi.org/10.1002/biot.202300685 Molecular Therapy Methods & Clinical Development, Volume 32, Issue 4, 12 December 2024, 101376