
Fully Characterizing a Manufacturing Process is Critical for Advancing Gene Therapies and Prioritizing Patient Safety
Gene therapies have positively impacted the lives of many patients and families around the world, and they continue to hold promise for those suffering from devastating genetic diseases. I am proud to have led the chemistry, manufacturing and control (CMC) efforts for a globally approved gene therapy for spinal muscular atrophy (SMA) that has now successfully treated more than 3,000 patients worldwide.i In the case of SMA Type 1, children with this most devastating form of the rare genetic disease are not likely to live to age two.ii Thanks to gene therapy, those children are now celebrating birthdays and milestones that were previously impossible. I share this as an example of the truly incredible potential gene therapies and other advanced medicines have for patients and families.
Currently, there are approximately 16 gene therapies (including genetically modified cell therapies) approved by the United States Food and Drug Administration (FDA).iii Innovators, regulators and patients all want to see that number increase exponentially in the near term, and a crucial part of achieving that goal is ensuring optimal safety profiles for gene therapies. It is well understood that manufacturing a therapeutic product with an excellent impurity profile contributes to improved safety and patient outcomes. Commonly, manufacturers concentrate on optimizing full versus empty capsid ratios in a final drug product. However, paying attention to capsids alone will not wholly address this issue. What’s missing from that calculation is a focus on the comprehensive impurity profile, including a priority around functional full capsids. Fully characterizing a manufacturing process to understand what’s driving relative levels of impurities is critical. Ultimately, designing a process that maximizes the safety and quality attributes of a medicine could support an increase in the number of gene therapies reaching the market.

What are functional full capsids and why are they important?
When thinking about the purity of a gene therapy drug product, manufacturers and regulators generally consider a couple different indicators – percentage of full capsids and presence of other impurities. A full capsid contains a full genome sequence to ensure the product is therapeutically active. An empty capsid does not carry genetic material, and a partially filled capsid may contain fragments of DNA that are not therapeutically relevant and in some cases, may be detrimental to patients. A functional full capsid, however, provides the intended therapeutic benefit and contains minimal ineffective or suboptimal genetic content that could contribute to safety concerns. The distinction may seem minor but the capability to definitively separate full, partial and empty capsids helps to maximize product purity and potency, prioritize patient safety, and support a robust CMC package for regulators.
There’s no substitute for deep analytical experience
Traditionally, CMC efforts focus on identifying impurities, including capsid impurities, using one or two analytical tests. It is vitally important, however, to comprehensively assess the end product and consider exactly what is contained within the capsid. The Advanced Medicine Partners team is responsible for developing and qualifying analytical methods to support multiple commercial advanced medicine products. We leverage this deep experience to select an extensive set of orthogonal analytical methods and fully characterize the manufacturing process to ensure robust and reproducible unit operations. At Advanced Medicine Partners, we prioritize the full characterization early on in the product lifecycle and enrich for functional full capsids and reduced levels of impurities to ensure patients are receiving product that maximizes potency and minimizes safety concerns. See Figure 1.

All of the news, delivered with full-text to your inbox. For professionals discovering, developing, and marketing biopharmaceutical drugs.
FIGURE 1
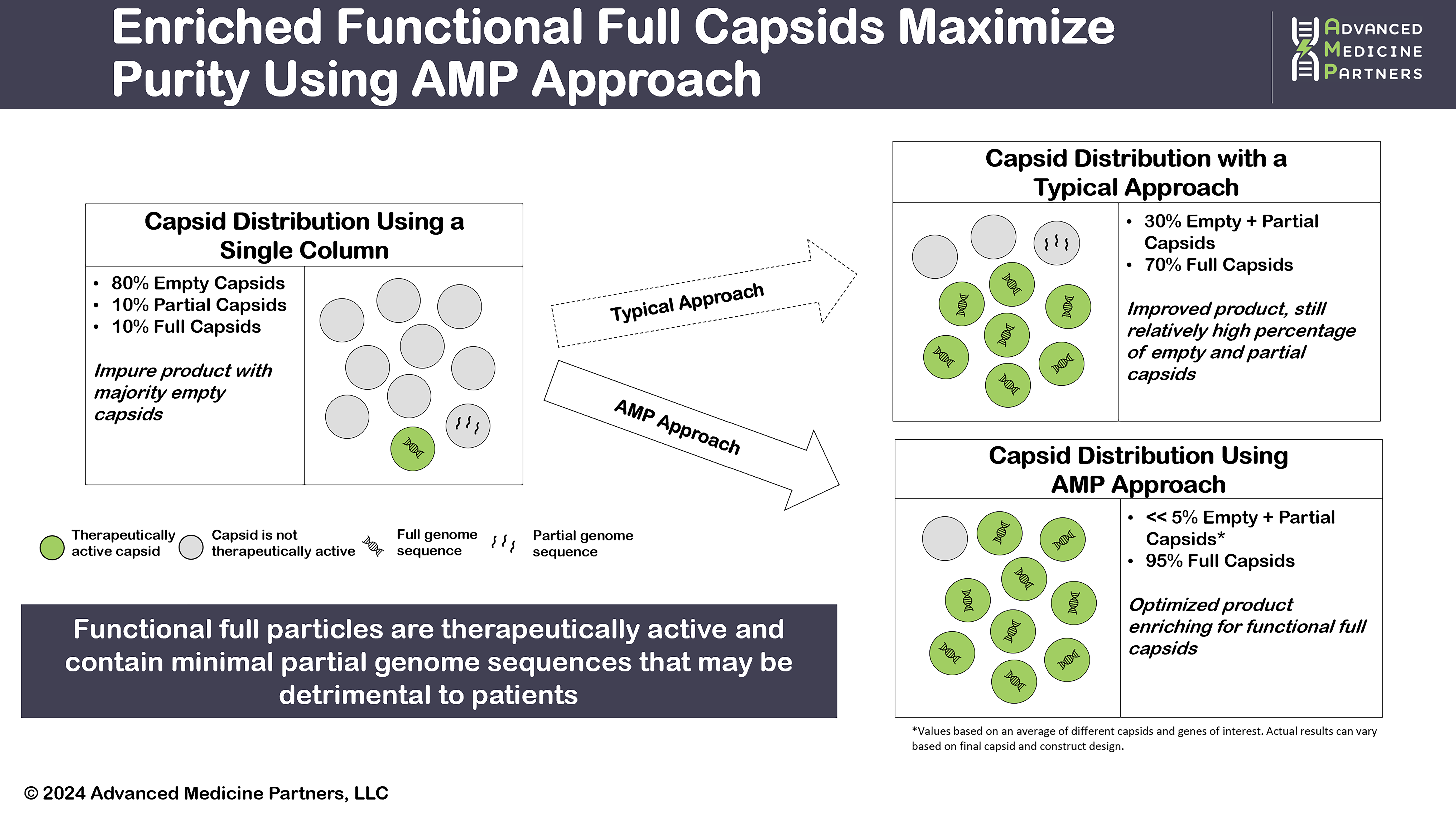
It’s Critical to Characterize Product Using an Orthogonal Set of Methods
Several analytical methods are widely used to identify full, empty and partial capsids. One is the calculation of vector genome titer by ddPCR or qPCR versus total capsid quantification by serotype-specific ELISA. This is a commonly used technique, and while it can detect full or empty capsids, it cannot differentiate functional full from partially full capsids. Transmission electron microscopy (TEM) and high-performance liquid chromatography (HPLC) are other techniques used for characterizing capsids. An advanced and effective method is density-based separation through analytical ultracentrifugation (AUC). Including AUC, Advanced Medicine Partners employs several orthogonal analytical methods, strategically using them at appropriate stages of the manufacturing process, to fully characterize product and precisely identify functional full, partial and empty capsids.
By strategically using multiple analytical methods, Advanced Medicine Partners can provide partnered companies with a superior therapeutic product with maximum purity and potency. See Figure 2
FIGURE 2
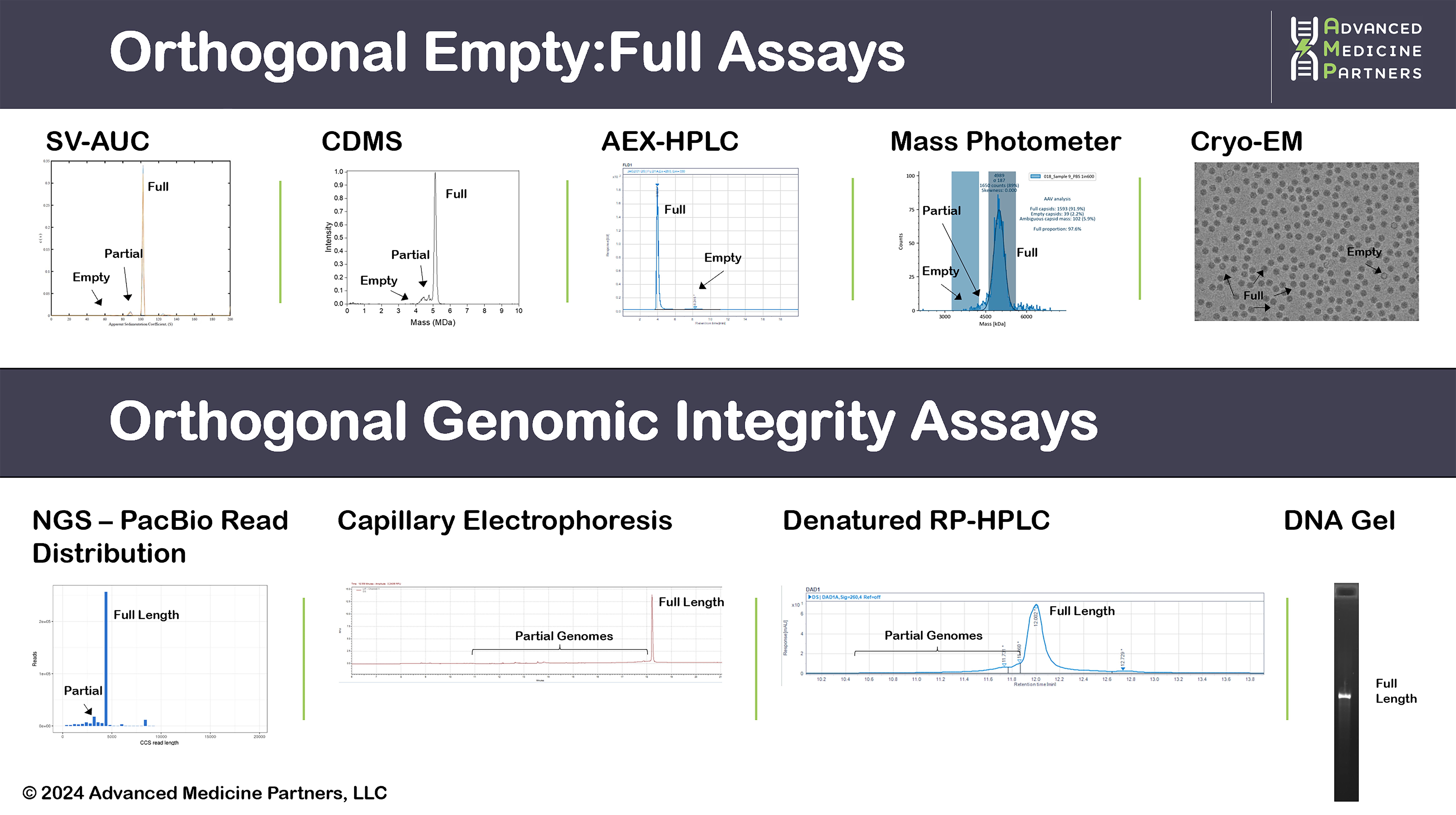 To increase the approvals of gene therapies with life-changing potential, we need to set a new standard for manufacturing and analytical testing. Fully characterizing a manufacturing process and product allows sponsors to develop a filing package that provides regulators with the robust data they expect. Increased product purity will support improved patient outcomes, and patients deserve the best of what’s possible. Identifying and enriching for functional full capsids is pivotal for maximizing product purity and increasing potency of gene therapies. At Advanced Medicine Partners, we have mastered the capability to fully characterize a product to ensure partnered companies receive drug product that minimizes impurities. We are eager to use this knowledge and deep experience to progress gene therapy manufacturing and accelerate gene therapy programs to the clinic and beyond.
To increase the approvals of gene therapies with life-changing potential, we need to set a new standard for manufacturing and analytical testing. Fully characterizing a manufacturing process and product allows sponsors to develop a filing package that provides regulators with the robust data they expect. Increased product purity will support improved patient outcomes, and patients deserve the best of what’s possible. Identifying and enriching for functional full capsids is pivotal for maximizing product purity and increasing potency of gene therapies. At Advanced Medicine Partners, we have mastered the capability to fully characterize a product to ensure partnered companies receive drug product that minimizes impurities. We are eager to use this knowledge and deep experience to progress gene therapy manufacturing and accelerate gene therapy programs to the clinic and beyond.
To learn more about Advanced Medicine Partners’ capabilities and services, visit www.ampgtx.com or email us at partnership@ampgtx.com.
References:
iNovartis. 2023. Q4 2022 Results Investor presentation [PowerPoint Presentation]. Available at: https://www.novartis.com/sites/novartis_com/files/q4-2022-investor-presentation.pdf. Accessed December 2023.
ii Kolb SJ, Kissel JT. Spinal Muscular Atrophy. Neurol Clin [Internet]. 2015 Nov [cited 2017 Oct 2];33(4):831–46. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4628728
iii American Society of Gene and Cell Therapy. 2023. Q3 2023 Quarterly Data Report [PowerPoint Presentation]. Available at: https://asgct.org/global/documents/asgct-citeline-q3-2023-report.aspx. Accessed December 2023.