How Australia Delivers Rapid Start-up and 43.5% Rebate for Early Phase Oncology Trials
About Avance Clinical
Avance Clinical is an Australian owned Contract Research Organisation that has been providing high-quality clinical research services to the local and international drug development industry for 20 years. They specialise in working with biotech companies to execute Phase 1 and Phase 2 clinical trials to deliver high-quality outcomes fit for global regulatory standards.
As oncology sponsors look internationally to speed-up trials after unprecedented COVID-19 suspensions and delays, Australia, which has led the world in minimizing the pandemic’s impact, stands out as an attractive destination for early phase trials. This in combination with the streamlined regulatory system and the financial benefits including a very favourable exchange rate and the R & D cash rebate makes Australia the perfect location for accelerating biotech clinical programs.
 Yvonne Lungerhausen, CEO, Avance Clinical
Yvonne Lungerhausen, CEO, Avance Clinical“When we advise sponsors that Australia has a 43.5% rebate on clinical trial costs it becomes a straightforward decision for them to initiate their trials in Australia. Australia’s clinical sites and infrastructure are very much open for business,” said Avance Clinical CEO Yvonne Lungerhausen.
Approximately 96% of our work is supporting small to medium-sized biotechnology companies executing Phase 1 and Phase 2 trials. Our client base currently extends across North America, Canada, New Zealand, Europe and Asia.

All of the news, delivered with full-text to your inbox. For professionals discovering, developing, and marketing biopharmaceutical drugs.
“Working with the Avance Team has helped us to develop and complete high quality and compliant clinical studies. I especially appreciate their penchant for communication, quality and pragmatism.” – Sr. Vice President, Regulatory, Quality & Clinical Affairs, Atossa Genetics, Inc
“We offer 20-years of experience in the CRO sector servicing biotech companies from around the globe. The demand for our services is driven by our scientific and research excellence, streamlined regulatory environment, advanced healthcare system,” said Yvonne Lungershausen.
A review of data from TrialTrove reveals that to date there are approximately 100 Phase 1 trials ongoing or planned to be initiated in Australia by biotech’s in 2020 compared with over 500 Phase 1 trials planned to initiate in the US. While Australia performs well in this space on a global stage there remains significant opportunity for biotechs to take advantage of Australia’s world-class early phase trials ecosystem.
Learn more and register to watch our Oncology capabilities presentation.
Avance Clinical is perfectly placed to partner with biotechs for the early clinical development of their oncology assets
 Gabriel Kremmidiotis, Chief Scientific Officer, Avance Clinical
Gabriel Kremmidiotis, Chief Scientific Officer, Avance ClinicalMore than 20% of Avance Clinical’s studies are in the Oncology therapeutic area. After conducting more than 30 studies over the past 4 years and managing a total of over 5000 patients this experience has enabled Avance Clinical to develop strong site and investigator relationships and best in class capabilities in the conduct of single centre Phase 1 to multi-centre Phase 2 trials.
“Australia is an ideal hub for early phase oncology trials and many international biotech and pharma companies launch early phase clinical evaluation of their programs in Australia,” says Chief Scientific Officer, Gabriel Kremmidiotis.
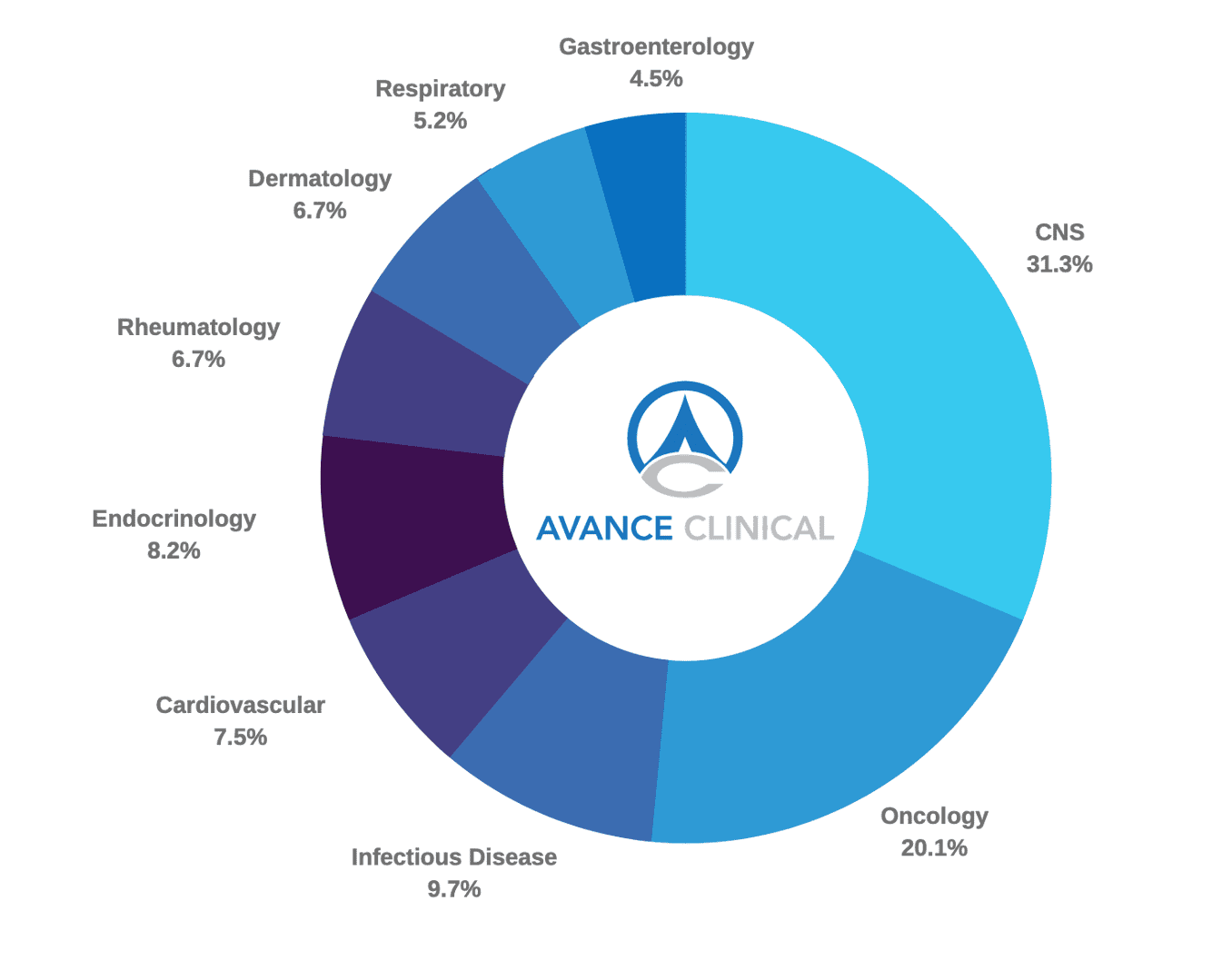
Breaking this out further Avance Clinical’s Oncology therapeutic indication experience includes:
- Breast Cancer
- Acute Myeloid Leukemia
- Solid Tumors
- Gynecologic Malignancies
- Gastric Cancer
- Diffuse Intrinsic Pontine Glioma (DIPG)
- Glioblastoma Multiforme
- B-cell Lymphoma
- Colorectal Cancer
- BRAF mutation-negative Metastatic Melanoma
- Metastatic Renal Cancer
- Mammographic Breast Density
- Acute Leukemia
- Upper Limb Secondary Lymphedema
Leveraging deep experience in Oncology clinical trials and the latest innovation in study design
Based on Avance’s’ experience in these indications and having managed many early Phase oncology trials in the past 4 years Avance is able to recommend to clients a number of early-phase study design elements that are considered “best return on investment” for FIH products. Such designs incorporate the following key elements:
- An accelerated dose-escalation part, included in the dose-escalation component of a Phase I study. This design accelerates dose escalation through the initial subtherapeutic doses.
- Inclusion of a combination arm to evaluate the safety of the investigational agent when combined with an approved agent. Evaluation of the combination is included in the dose-escalation phase with the combination off-set to the monotherapy of the investigational agent by one dose cohort.
- Inclusion of a sequential monotherapy arm in randomised Phase 2 trials where the new investigational product is being evaluated in a combination setting involving the standard of care (SOC) (e.g. Keytruda plus/minus the investigational agent). This design allows the acquisition of data on the investigational product used as monotherapy while only using two treatment arms.
“Avance’s team and network of experienced clinical research investigators and sites have the breadth and depth of expertise necessary to successfully conduct Phase I and Phase II oncology trials across all tumour types, in tumour-specific or molecular-target-specific settings,” comments Gabriel Kremmidiotis, Avance Clinicals Chief Scientific Officer.
Avance has embedded oncology expertise at all levels and departments to ensure the complexity of each oncology trial is understood, and trial objectives are delivered.
Unpacking the Australian Advantage
The fast-regulatory approval environment, the availability of key opinion leader expertise and high-quality clinical research sites in Australia provides an ideal setting for the fast and quality execution of clinical trials.
The world’s fastest Study Start
The Australian regulatory body for clinical trials, the Therapeutic Goods Administration (‘TGA’), offers two schemes for conducting clinical trials in Australia; the CTN Scheme and the CTX Scheme. Most clinical trials initiated in Australia are under the CTN Scheme. The CTN Scheme involves a simple notification process following Ethics Committee (hospital or site network-based review committees) approval for the study. It is important to note that an active IND is not required to initiate trials in Australia under the CTN or CTX Scheme.
Key points include:
- Site Initiation Visit (SIV) and Study Start can be achieved in 5-6 weeks.
- Working within this robust and efficient framework expedites the process of running a clinical trial.
- No IND required for clinical research trials
- Full GMP material is not mandated for Phase I clinical research trials
Rebate of Clinical Trial Costs
Administered by Innovation Australia and assisted by AusIndustry and the Australian Taxation Office (ATO), The Australian Government’s Research and Development Tax Incentive has made Australia an attractive destination to take advantage of R&D initiatives.
Key points include:
- Ideal for companies with an aggregated turnover of less than $20 million that are in a tax loss position.
- Rebate up to 43.5 cents from every dollar spent on R&D
- Manage cash-flow
- Improve R&D project and knowledge management
- Ensure compliance with R&D legislation
Working with Ethical Review Process for rapid Start-up
Selection of appropriate HRECs for early phase oncology trials in Australia is of primary importance in attaining a fast start-up and trial completion. The Avance team uses its experience with a network of over 30 sites in Australia in preparing HREC approval plans which aim to fast study start with a minimal number of submissions. The scientific and ethical review of clinical trials in Australia is managed by the Human Research Ethics Committee (HREC). To ensure a fast start-up of clinical trials, Avance Clinical selects sites that utilise rapid HREC. All study documentation is submitted electronically via eProtocol 2 weeks before a meeting. From submission of the application to communication of the review decision, these HREC have an approximate turnaround time of 20 working days. Due to this streamlined regulatory and ethical review process, Avance Clinical can achieve a Site Initiation Visit (SIV) and commence a study within 5 – 6 weeks of submission to the HREC.
“Avance’s scientific and medical affairs team assists clients to navigate regulatory submissions and ethics study approval through the provision of advice relating to the content of Investigators Brochures and proposed study designs and protocols. This ensures speed of ethics review and approval,” says Gabriel Kremmidiotis, Avance Clinical’s Chief Scientific Officer.
Case Study: Avance Clinical – The Clinical Trial Checklist – How we work
Site Selection and Activation
Avance Clinical has a significant database of clinical research sites that we approach for oncology studies. Our feasibility strategy involves initial reach out to Key Opinion Leaders (KOLs) in Australia (and if applicable, other countries through our partner CROs). Our aim is to find influential KOLs that actively perform for studies to select as our key sites. The impact of this is that other sites will be drawn to participation based on the involvement of the KOL.
Following the initial reach out and expression of interest by the investigator, we send the investigators information and a questionnaire for the study and engage them through either a phone call or in-person conversation. If the interested investigator has the targeted patient population, we follow up with the conduct of a pre-study site assessment visit to confirm the qualification of the site.
Managing Patient Accrual
Our strategy for managing patient recruitment includes prospective, dynamic and retrospective aspects.
We prospectively generate accrual estimates obtained during initial site feasibility. The process involves consultation with prospective study investigators on the patient population to be accrued to the study as well as consideration of the proposed study eligibility criteria and any other concurrent competing trials.
Furthermore, our projections for patient recruitment and our estimate of site number requirements take into consideration timeframes and accrual rates of studies we have managed in the past, plus benchmark recruitment data from TrialTrove as well as public domain data available for completed studies of similar design, patient number and disease.
Biometrics & Medical Writing Considerations
Avance Clinical has developed robust biometrics processes for key operational aspects of studies including for the data management, biostatistics and medical writing deliverables. The Avance Clinical data management and medical writing teams provide a nimble and fully in-house solution. The advantage is streamlined delivery including the following indicative timelines:
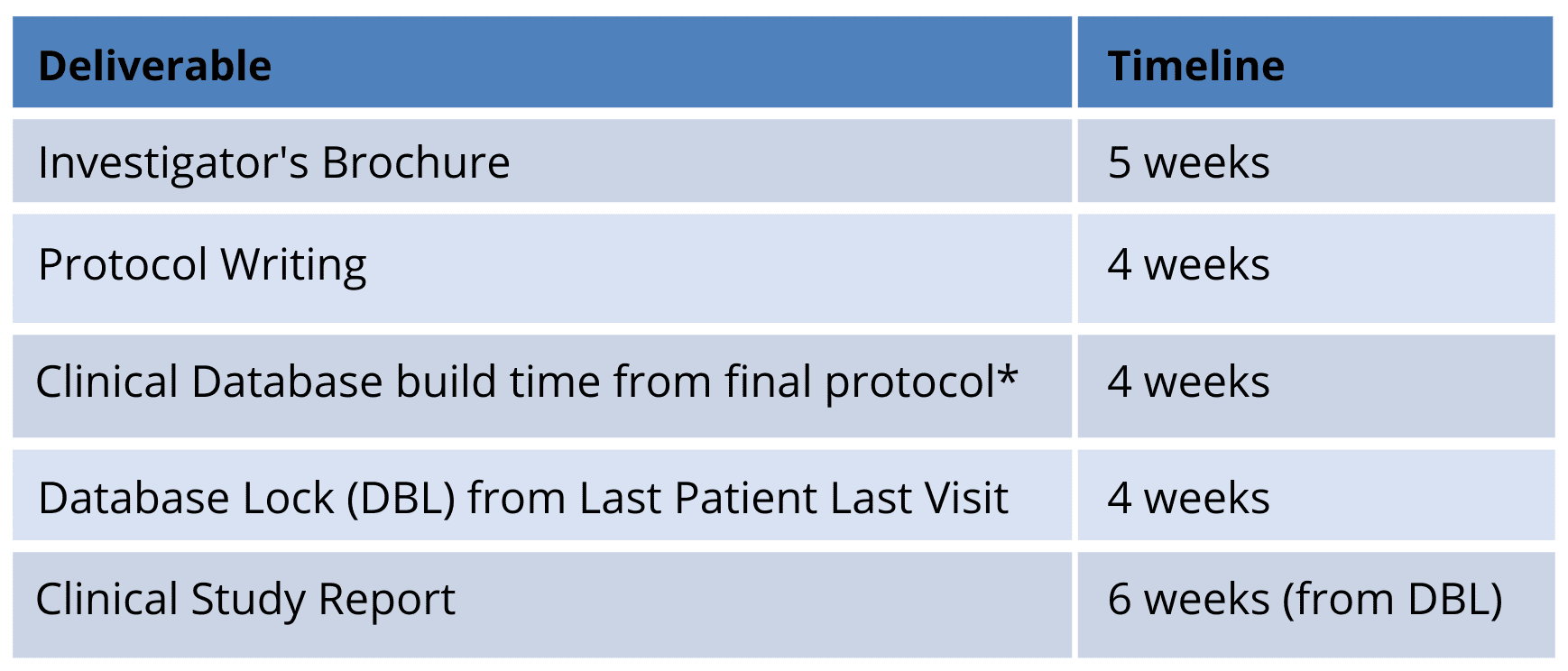
*This timeline relies on timely responses from all stakeholders reviewing the draft Case Report Form. However, the experienced Avance Data Management team will drive this process.
- The Avance Clinical biostatistics team is also a full in-house service and co-located with the data management team in the Adelaide (Australia) office. This creates adaptability and flexible delivery due to their increased ability for real-time communication and problem solving.
- Following the provision of the draft Tables Listing and Figures (TLFs) to our clients for review, the completion of the final TLFs and the Clinical Study Report is reliant on the review time from the client team.
- Our Statistical and Pharmacokinetics Services team services conform to the regulatory-compliant CDISC model, with a focus on the SDTM and ADaM standards and their supporting documentation. We ensure full data traceability and track value level metadata so that our supporting Define.xml complies with Pinnacle21 validation and our deliverables are submission-ready for your clinical research trial.
The Clinical Project Team
Avance provides each trial with a dedicated Clinical Project Manager (CPM) to ensure that milestones are reached, timelines are met and that our clients receive effective communication and ongoing status reports.
From the first conversation to the delivery of the final report, our clients are fully informed throughout the process; irrespective of project complexity.
Our CPM will be involved in all aspects of clinical trial management, including:
- Site feasibility assessments, investigator selection, and site selection
- Study design and Protocol review
- Development of patient information and consent forms
- Ethics Committee submissions
- Review of eCRF design and development
- Oversight of site monitoring activities & cross-functional management.
The Avance CPM is also the single point of contact for our clients. Following the award of the study to Avance, the CMP will organise a study Kick-Off meeting involving both the client team and key Avance team assigned to the project. The CPM then liaises with all Avance teams involved throughout the study to manage the study timelines, deliverables and overall communications. The organisational chart below is indicative of the project team set-up.
The Avance team is a highly experienced, streamlined team with a ‘can-do’, solution focussed mindset including a very experienced team of Project Managers.
Avance Clinical has the therapeutic experience, flexible and robust quality processes and experienced team necessary to support biotech and pharma companies to expedite the development of much needed new therapies and we welcome the opportunity to discuss your program further and explore how we can best support your development needs.
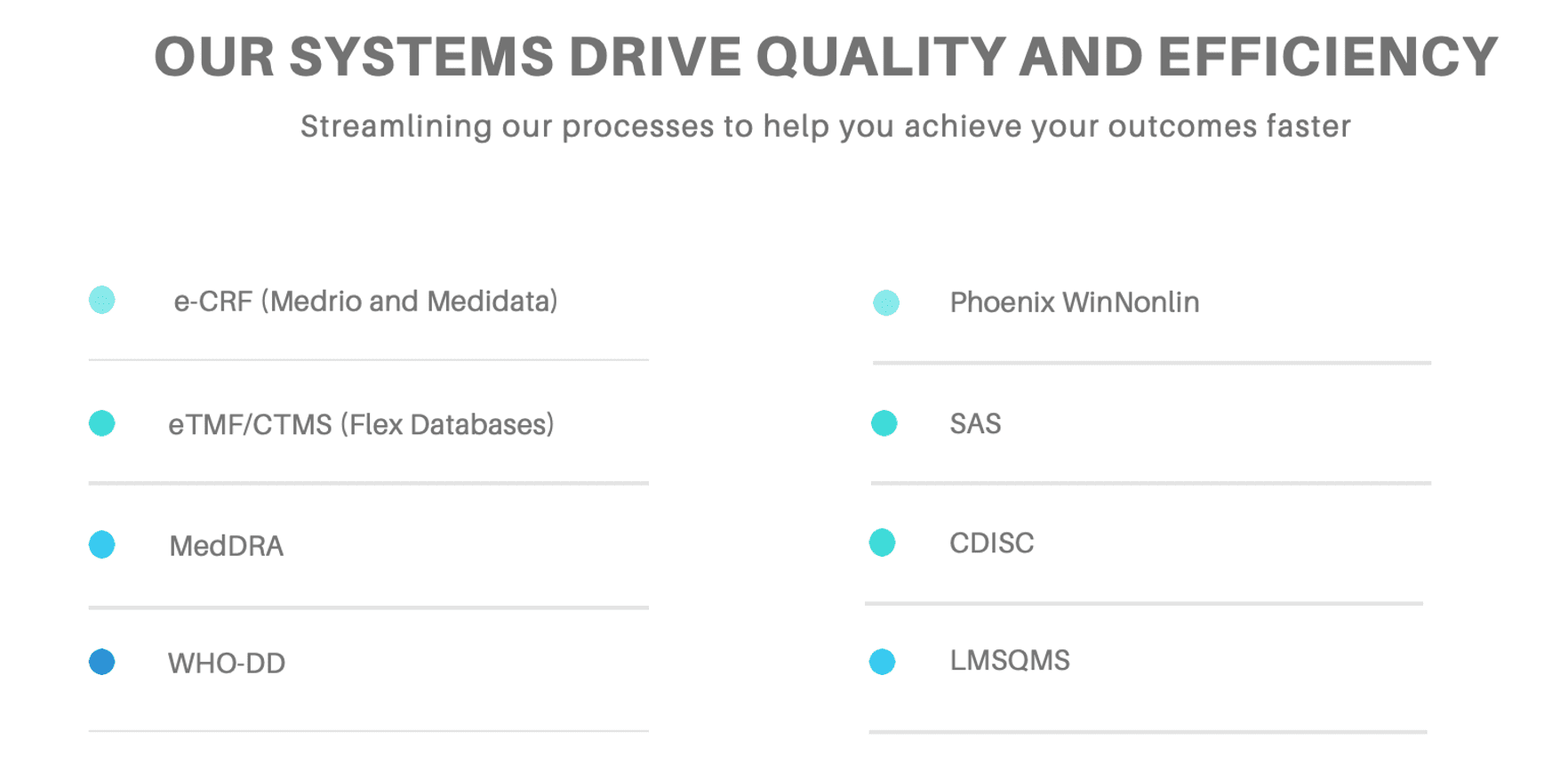
Robust Systems that Drive Quality
Avance understands that the success of clinical program delivery is also underpinned by having robust systems that drive quality. From the ability to develop eCRFs utilising Medrio or Medidata, the review and analysis of data utilising MedDRA and WHO-DD for coding, Phoenix WinNonlin for Pharmacokinetic and Pharmacodynamic analysis, SAS for statistical analysis and CDISC capabilities Avance has a full suite of best in class systems to deliver quality outcomes. Program delivery is also supported by the Flex Databases Clinical Trial Management System and eQuality Management System and eLearning Management System from MasterControl.