How Patients with Epilepsy Benefit from Real-World Data
Amanda Shields, Principal Data Scientist, Scientific Data Steward
Keith Wenzel, Senior Business Operations Director
Andy Wilson, Scientific Lead
Real-world data (RWD) has the potential to transform the drug development industry’s efforts to predict and treat seizures for patients with epilepsy. Anticipating or controlling an impending seizure can significantly increase quality of life for patients with epilepsy. However, because RWD is secondary data originally collected for other purposes, the challenge is selecting, harmonizing, and analyzing the data from multiple sources in a way that helps support patients.
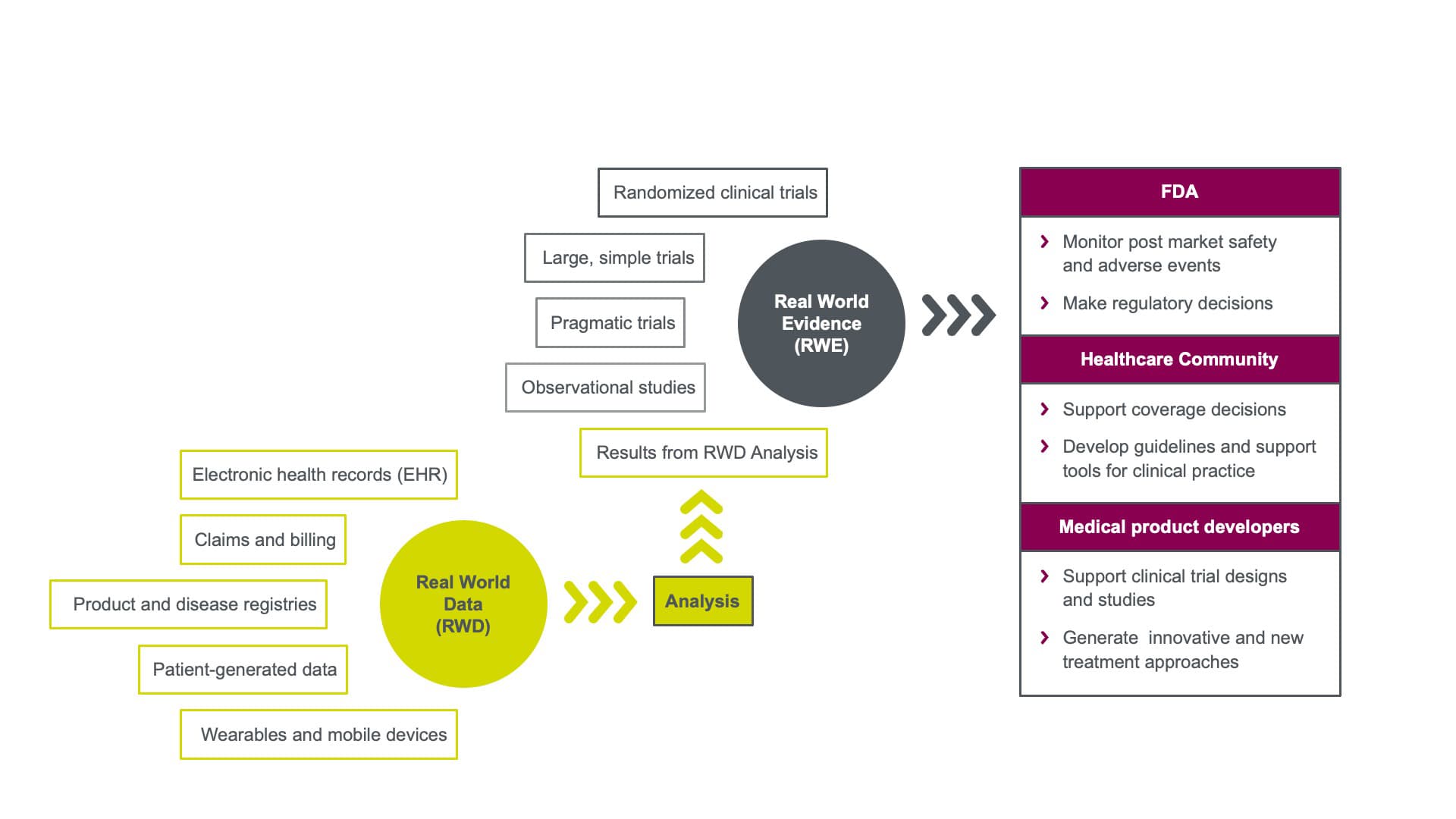
What is RWD versus RWE?
According to the U.S. Food and Drug Administration (FDA), RWD are “data relating to patient health status or delivery of health care routinely collected from a variety of sources.¹” Real-world evidence (RWE) is “clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of RWD.” Figure 1 illustrates how RWD supports RWE and how that evidence may be used to support different segments of the life sciences industry.
RWD is all about context
RWD can potentially help researchers answer questions quickly and efficiently, and for a broader and more representative global patient population than randomized clinical trials (RCTs) alone. By design, RCTs eliminate much of the real-world context, such as dynamic treatment decisions, to ensure data are collected in the same way at every site. RCTs are carefully designed to control bias and reduce any variations that could influence study results. Patients must meet strict inclusion/exclusion and adherence criteria, so trial results may not always be generalizable to the broader patient population or reflect how treatments will be used in ways that fit with their lives.
For RWD, the who, what, when, where, why and how data are generated provide us with important context. For example, timestamps on seizure data collected from intracranial EEG readings may be important data points for dynamically forecasting seizure events in real time. Seizure data collected from patient diaries can provide evidence of disease progression that may be otherwise difficult to capture. RWD and RWE can further:
- Expedite patient recruitment and enrollment
- Expedite patient access to treatment
- Significantly supplement data collected from RCTs
- Better inform how to use treatment in clinical practice
- Personalize treatment for patients, based on their specific profile
How can RWD be used in clinical studies to improve quality of life for patients with epilepsy?
Epilepsy is a disease that occurs in almost 1% of the population worldwide² and is characterized by recurrent seizures. Although many patients can control their disease with regular medications, about one-third of patients cannot.
By using data to understand patient-level patterns and cycles of seizures, sponsors can compare apples to apples more easily. “You can’t run all the RCTs you may want to make direct comparisons between therapies or combinations of patient profiles or characteristics,” says Amanda Shields, Principal Data Scientist, Scientific Data Steward at Parexel. “For example, observational studies in network meta-analysis (NMA) can be used like a round robin tournament—to quantify indirect comparisons.”
Accurately predicting seizures helps sponsors determine which treatments work better for which patients and provides clinicians tools to devise more personalized treatment plans. Removing the unpredictability of and controlling seizures helps improve quality of life for patients.
What is the “right” data for epilepsy studies?
As of this article’s publication, the FDA has not issued guidance on all aspects of RWD or RWE. At the end of 2018, the FDA released a draft framework for a new Real-World Evidence Program in which it proposes to use the RWE Program to guide generation of data in support of approval for new indications or to help support post-approval study requirements.³ Under this framework, RWD can be used to supplement traditional clinical trial data and improve the efficiency of these trials. According to the RWE framework, one criterion for evaluation by the FDA is if the RWD selected is fit for use to generate data for product effectiveness decisions.
At Parexel, we conduct a robust feasibility process which begins with the question the sponsor is trying to answer to ensure we are collecting the “right” data for the specific epilepsy study. Let’s say Sponsor A wants to evaluate the effectiveness of a drug on preventing acute seizures in patients with uncontrolled or incompletely controlled seizures on management therapy. One important source of data will be collected from our patient who is wearing a non-invasive sensor like a smartwatch or other device. Wearables can provide significant new predictive features. We will combine the predictive data with historical data from patient diaries and electronic health records (EHRs) to develop a complete picture.
We then collect, harmonize, and analyze these data, which helps us reliably predict seizures, alert the patient when he may be at risk of an acute event, and prompt him to take the medication.
What are the challenges with using RWD for epilepsy clinical studies?
There can be challenges with using RWD for any clinical study. Epilepsy studies present some unique challenges because the disease is not treated in a single care setting—it might be treated in a hospital, primary care, or specialist setting. Not all EHRs are integrated, so there may be gaps in the data. In the example we mentioned earlier, our patient may not like his wearable or may forget to maintain his patient diary. Data accuracy can be affected if we have excessive data gaps due to any or all these issues.
CHALLENGES WITH RWD
- Lack of prescribed regulatory framework
- Accurate, timely and useful data
- Linking data from multiple sources or settings
- Deidentified data
- Patient consent for using their data beyond a clinical trial setting
- Where data is stored and how to access it
- Analytic assessment and remedies for multiple sources of bias, e.g., selection bias and immortal time bias
- Small versus large data sets
Conclusion
RWD, used appropriately, can help us put together the puzzle pieces that reveal a very personal story about epilepsy patients. Patients can benefit from the synthesis of all their streams of data, including their own perspective, analytically integrated into both personal stories and evidence that can speed the development of treatments that are more precise and personalized to the needs of each patient.
Âą U.S. Food and Drug Administration: Real-World Evidence, 30 Nov 2020, https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence
² Banerjee, P. N., Filippi, D. & Allen Hauser, W. The descriptive epidemiology of epilepsy- a review. Epilepsy Res. 85, 31–45 (2009)
³ PharmExec: Framework for FDA’s Real-World Evidence Program, 31 Jan 2019, https://www.pharmexec.com/view/framework-fda-s-real-world-evidence-program-0