
Is Australia the next Global Destination for Phase 2?
Avance Clinical is the leading Australian CRO for biotechs, and a Frost & Sullivan Asia-Pacific CRO Market Leadership Award winner. Avance Clinical is a full-service CRO with 24-years of experience, led by CEO Yvonne Lungershausen who is recognised as co-founder of Australia’s clinical research industry. The company has deep expertise, as well as extensive partnerships with technology leaders including Medidata, Oracle, and Medrio to deliver the region’s best clinical trial services to its biotech clients. Avance Clinical grew more than 57% in 2020 and has more than doubled staff numbers.
Please visit our website at https://www.avancecro.com/ or email us at enquiries@avancecro.com for more information.
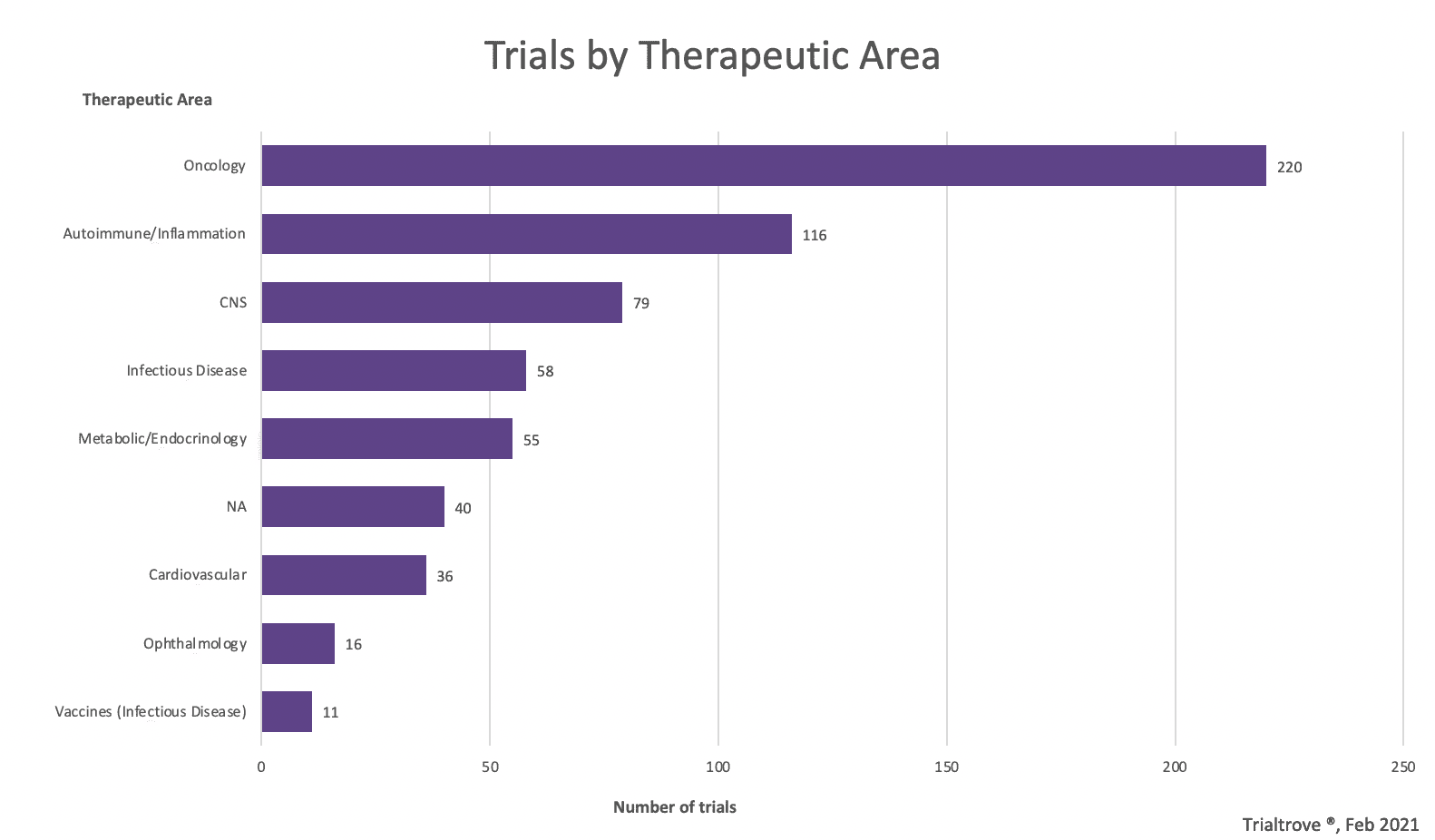
According to TrialTrove, there have been 1,411 industry sponsored clinical trials recruiting in Australia and New Zealand since 2019, with 330 currently planned. Of these there are 144 Phase 2 studies and 114 in Phase 3 and 4 studies.
A year ago Australia was largely known predominantly for its early phase capabilities. However much has changed with Australia becoming the “COVID-19 free” envy of the world. Australia regularly records zero new daily COVID-19 cases, backed by the world’s leading contact tracing systems, mandatory travel quarantine, and strict borders.
Sponsors are now staying beyond their Phase I studies and starting their Phase 2 studies in Australia as they discover Australia is an exceptional medical research location, especially amid a global pandemic.
Having conducted several Phase I studies in Australia it made sense to continue in the country with our Phase II program, particularly given the high quality support we have received from our CRO.
Atossa Therapeutics Inc
The Australian Shift Here to Stay
In addition to the “COVID free” status, Australia outperforms in all quality and compliance metrics, and has demonstrated it can deliver the patients needed via innovative recruitment methods and off-shoring partnerships with powerful and proven site networks in Eastern Europe and Asia.
A key reason for continuing a study in Australia is the highly-regarded data integrity. Australian clinical research data is regulatory ready meaning it is accepted by all major regulatory authorities around the world.
 Yvonne Lungershausen, CEO, Avance Clinical
Yvonne Lungershausen, CEO, Avance Clinical“Increasingly clients are seeing the advantages of not only conducting their Phase 1 studies in Australia but then expanding into Phase 2 multi-sites starting in Australia first with rapid start-up, then out to other regions.
Sponsors can leverage their strong relationships with PIs and KOLs for their Phase 2 activity.
The Australian clinical and medical infrastructure lends itself to highly effective patient recruitment.”
Yvonne Lungershausen, CEO, Avance Clinical
Attractive Government Rebate
Assured continuity, speed, and leveraging the Australia Government’s 43.5% rebate incentive mean sponsors are chosing to stay in Australia for their later Phases, even to Phase 4.
There are some key therapeutic areas that are ideally suited for Australia clinical research including oncology, central nervous system, ophthalmology, allergies and dermatology.
Patient Recruitment
Unlike most other parts of the world right now, patients in Australia can visit clinics and hospitals for study appointments without fear of contracting COVID-19. Also, medical facilities are at their pre-COVID-19 patient numbers so are open for business as usual.
 Gabriel Kremmidiotis, PhD, BSC Hon., Chief Scientific Officer, Avance Clinical
Gabriel Kremmidiotis, PhD, BSC Hon., Chief Scientific Officer, Avance Clinical“According to a recent report published by MTPConnect, there are 37 clinical trial networks operating in Australia.
These networks are led by highly experienced clinicians and clinical research staff offering a trial-ready infrastructure with access to patient groups in a wide range of therapeutic indications”
Gabriel Kremmidiotis, Chief Scientific Officer, Avance Clinical
Australia is home to some of the most advanced digital recruitment companies in the world. Companies that offer digital micro-matching and screening to speed up the enrollment process at sites. Australian sites work closely with these innovative firms, as well as having their own recruitment tools tapping specialist physician referrals and patient advocacy groups.
“Plexus Research, a Site Management Organisation, is building a community based clinical trial network in Australia to enable Sponsors to conduct trials across Australia. To support Phase II, in the time of Covid, Plexus has a decentralised clinical trial operating model that enables Sponsors and CROs to reach and conduct trials nearer to trial participants and enabling better outcomes and lower costs.”
Suhit Anantula, Founder & CEO, Plexus Research
In addition, companies like Avance Clinical have collaborations with off-shore sites in especially high population regions in Asia and Europe. One of these is Cromos Pharma which has access to 2,000+ sites in Eastern Europe. Avance Clinical and Cromos Pharma have successfully collaborated on studies where there is local site and patient involvement and other functions such as medical writing, data management and statistical analysis remains in Australia and expanded rapid recruitment happens in Eastern Europe.
The Cost of Moving
Moving the next Phase of a study to a new location rather than staying with the same CRO and sites into Phase 2 can cost valuable time and hundreds of thousands of dollars.
When the stakes are high and costs can spiral it makes sense to maximise the time and cost advantages of the Australian environment to deliver high quality, regulatory ready data.