
Is Your Technology Keeping Pace with Your Science?
Science and technology are evolving at an unprecedented pace. Great care is called for when gathering requirements and making choices for scientific informatics and software needs. There are few more vexing challenges than inheriting a poorly performing and entrenched legacy enterprise IT environment riddled with data and process silos. The cost, politics, and persuasion needed for change can overwhelm those who haven’t been shown the true value and potential upside. The European Commission and Price Waterhouse Cooper published The Cost of Not Having FAIR Research Data in 2019, showing that this shortcoming alone costs the European Union ~ € 26 billion a year. We suspect this figure may be low as our analysis shows the cost per researcher being kept busy “wrangling data” forty percent of their time is higher. Pursuing Digital and Insilco Model-First strategies become unattainable, along with the ability to fully leverage “Big Data.” Disjointed processes and siloed data systems contribute to excessive costs, reduced quality, and elevated risk. So, you should care because the cost, poor performance, inefficient processes are not only eroding your organization’s capabilities but are also limiting the achievements of your research and development teams.
The goal is more efficient scientific business processes, leading to a better quality of life for scientists/researchers, and the consumers we are working for. Until now, there are very few enterprise scientific IT platforms that can span the continuum of life and material science organizations (Discovery to Product). The flexibility, scalability, and ability to impose rigor on complex processes will drive reproducibility, compliance, and FAIR data and processes. This improvement will ultimately deliver reproducibility in science, better availability and use of Model-Quality Data, and smoother or even seamless knowledge and tech transfer.
L7 Informatics, Inc. has built one of the best scientific unified platforms on the market called L7|ESP™. With this, L7 has raised the bar for enterprise scientific software with its “No Code/Low Code” capabilities and its efficiency in gaining Composable Architecture.
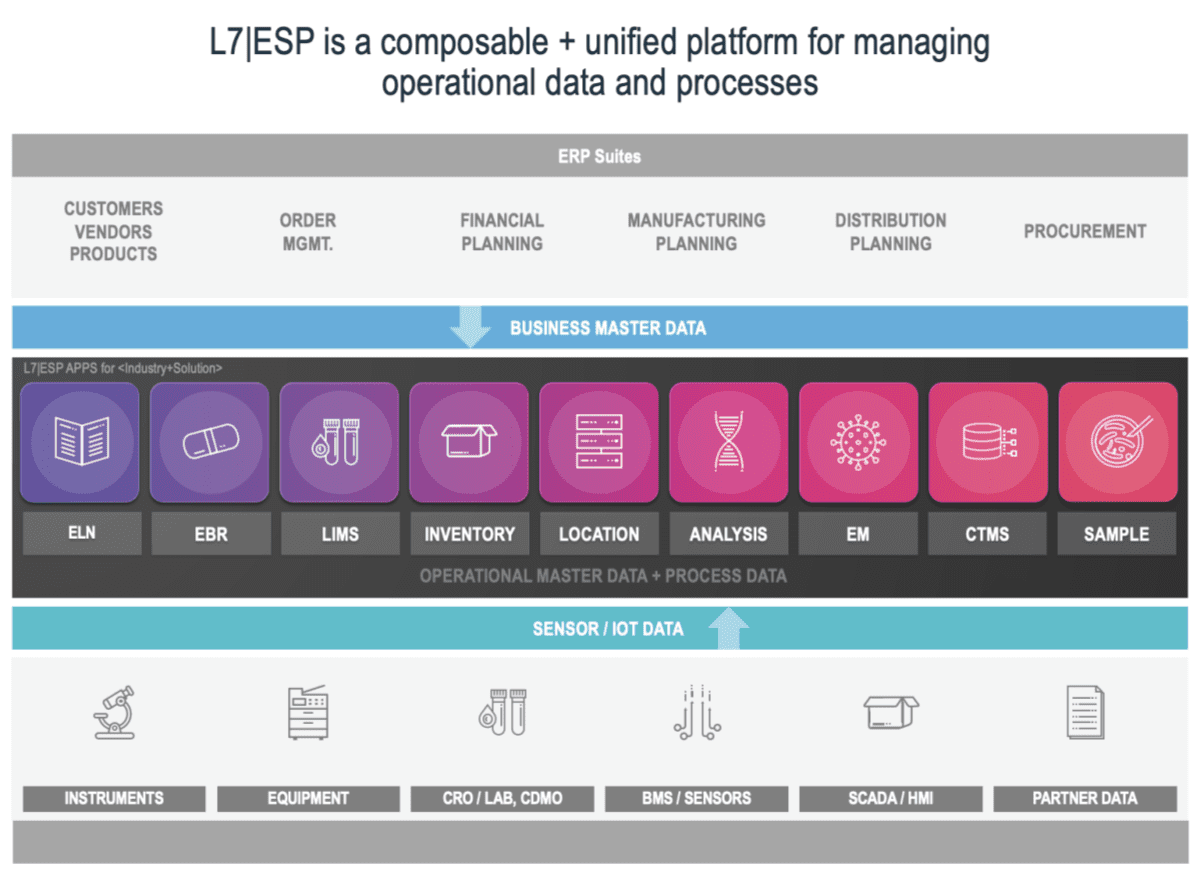
The composable architecture mentioned above, and the flexibility and preparedness it delivers, provide a blueprint for L7|ESP. It is a unified platform ecosystem that continually adds functionality (new business applications, instrument and equipment connectors, scientific content) and capabilities; with a rate of innovation that sets the standard in the field. Just last year, L7 Informatics was recognized by Gartner as Cool Vendor in Life Sciences and has been identified as a sample vendor in eight Hype Cycle reports. Recently L7 was featured and presented case studies with customers Cognate BioServices and Gradalis at ISCT and ASGCT respectively.
In drug discovery or therapeutics R&D, research typically proceeds in a dynamic state (with pockets of stability) then progresses to a more static state as a new product moves closer to manufacturing. Some other differences that impose exogenous constraints are the need for GXP and validation, which is on the Development, Clinical, and Manufacturing side of the house. The opportunity to reduce a company’s IT footprint is substantial and pressing. There are platforms available that can replace multiple legacy and antiquated tools and do it with speed, flexibility, and risk reduction. Composable approaches will help drive these efficiency gains by reducing costs associated with disconnected IT.
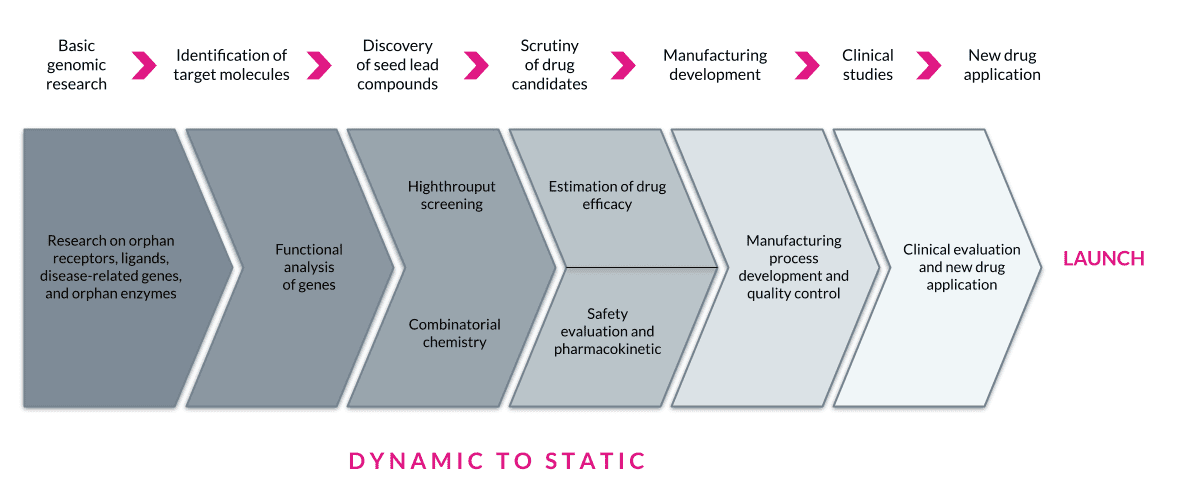
The current Biopharma and Healthcare verticals are not that simple for existing environments, vendor partners, and externalization and collaboration, all of which carry a vast amount of legacy IT. Brilliantly L7 integrates with existing systems and environments, allowing for a plug-and-play approach to evolving hybrid solutions while staying positioned to consolidate and simplify the overall IT footprint. Each step reduces unnecessary complexity, driving digitalization strategy in a coherent and manageable manner. This approach results in FAIR data and processes, opening the door to an Entity Life Cycle management approach that can drive innovation. A city planning analogy would be Boston versus Chicago. Have you ever been lost in Boston for hours trying to figure out how you will find your way back? (Thank the Gods for GPS.)
L7|ESP allows a well-written and architected tool to handle the complexities in major data-driven organizations. Whether it is gene editing data and processes, cell therapy, small molecule or large molecule development and manufacturing, companion diagnostics development, or agricultural sciences, the L7|ESP platform can handle the workflows due to its flexibility, scalability, and the reuse delivered by a composable architecture. Complex biologics development and manufacturing groups, including CDMOs, quickly see the value in this approach as their business processes can be become very variant due to the emergence of new modalities and precision medicine therapies.

What makes L7|ESP stand out as a unique Next-Generation platform are its reusable, FAIR-compliant building blocks. Scientific business processes are not only well defined but exist as a primitive or building block. Need to combine processes? No problem! Want to do this with data models? You can do it! And with the L7 approach data models, scientific workflows, instruments, applications, and services connectors are also part of the primitive framework. There is a growing element of COTS (Commercial Off the Shelf) but the ability to create new connectors becomes routine. Complexity in processes can be a precarious thing. If the complexity is due to poor processes, poor curation, and/or bureaucracy, only significant change management can address the problem. If the complexity is due to science and technology processes alone then L7 can reduce and manage it through its L7|ESP platform. The details of implementation will depend on the current and type complexity of each environment, and the role L7|ESP will play initially or in the months and years ahead. Remarkably, it does not take a year to configure the system, rather, it can become fully operational in weeks. This ultimately means lifesaving or altering products will get to market faster.
To learn more, please contact L7 Informatics for a full technical whitepaper on this subject.
John Conway is the Founder & CVO of 20/15 Visioneers.