Leading the Next CAR T Revolution in Cancer
David Chang, M.D., Ph.D
President, Chief Executive Officer and Co-Founder of Allogene Therapeutics
One of the greatest accomplishments for a scientist in their lifetime is advancing a new medicine that helps patients. There’s something incomparable in knowing that you’ve played a part in expanding the future of scientific discovery, in knowing that something once seemingly impossible is now a reality, and, most importantly, in knowing that you’ve created something that can help a fellow human being.
The danger lies in believing one new advancement is enough. As a medical oncologist, research scientist and biotechnology executive, I believe we must never rest or grow complacent. In my case specifically, that means progressing new therapies beyond previous accomplishments and breakthroughs made with the team at Kite Pharma.
Our work in cell therapy is far from done, as long as cancer continues to be a leading cause of morbidity and mortality, and patients continue to suffer. I believe we are on the cusp of the next revolution in cancer therapy – one built on the amazing foundation of autologous CAR T cell therapy. One that has the potential to make this type of therapy available to more people more quickly. My goal is to move CAR T into the fast lane with allogeneic.

Beginning the Revolution
At Kite, we had an ambitious goal of curing cancer through the development of groundbreaking cell therapies. We knew our lead program had the potential to change the paradigm of cancer treatment, but in the beginning, very few outside of Kite believed it was possible.
But we persisted and spearheaded the clinical development of Yescarta® (axicabtagene ciloleucel), the first autologous CAR T cell therapy to be approved by the U.S. Food and Drug Administration (FDA) for the treatment of adults with relapsed or refractory large B-cell lymphoma, a form of non-Hodgkin lymphoma (NHL).
Continuing the Revolution
Autologous CAR T therapy involves taking a patient’s own T cells and genetically engineering them to recognize and kill cancer cells. This therapy has produced remarkable responses in some patients for whom all other treatments stopped working, but as can be the case with the first product from a new modality, they come with certain limitations. One in particular is the source of the T cells from which an individualized therapy is produced.
This means that manufacturing and delivering a CAR T therapy can only happen after the cells have been taken from the patient. The wait of two to four weeks for patients, who already have failed multiple lines of therapy, can be significant particularly for patients with aggressive cancer. Additionally, some patients may not have enough T cells to expand and engineer.
It was time to change lanes and we were just getting started. Believing that recent advances in science, technology and genetic engineering had the potential to address the challenges of autologous CAR T therapy, Arie Belldegrun and I launched Allogene Therapeutics in April 2018 with the goal of creating “off-the-shelf” allogeneic CAR T cell therapies. While we have to overcome challenges to progressing new therapies, we believed in the potential of these therapies for on demand use and manufacture at scale. And because allogeneic CAR T therapies are created from T cells from healthy donors rather than from patients themselves, they have the potential to overcome the limitations of autologous therapies. At Allogene, we have created a robust pipeline of investigational allogeneic CAR T cell therapies in various stages of development to treat both blood cancers and the greatest challenge of all, solid tumors.
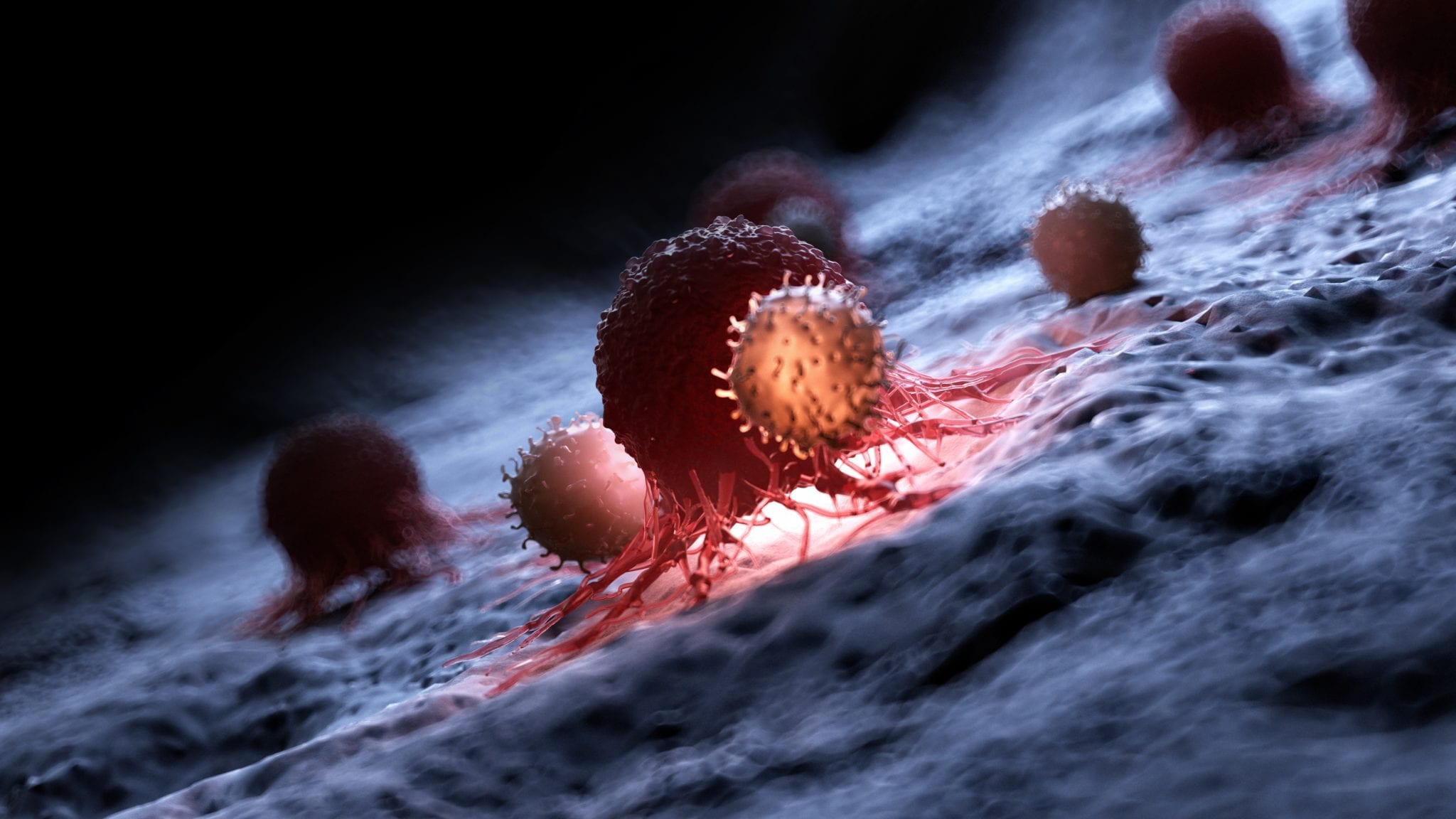
Illustration of a white blood cell attacking a cancer cell. Image provided by Allogene.
Click on the image to see the full-sized version
The Future of Cancer
Decades ago, a well-known computer scientist said, “The best way to predict the future is to invent it.” We know that the future is not dependent on a single invention, but rather an ongoing, ever-building process of innovation that we each have the power to propel in new, cutting-edge ways.
That’s why I’m hopeful for the future of cancer. I believe that allogeneic CAR T therapy represents the future. This nascent field is moving at lightning speed, but our ambition is to catch lightening in a bag.
We have made important first steps toward establishing allogeneic CAR T cell therapy as a viable platform. As we approach our one-year anniversary as a public company, our goals have not changed. We remain 100% focused and committed to evolving, refining, progressing and advancing the science of cell therapy. We must find balance between perfectionism and practicality as we aim to bring the next revolution in cancer treatment to patients in need. And on a highway filled with individuals who are motivated by improving patient lives, we will continue to build the body of research, we will continue to progress new treatments, and we will not rest until cancer has been cured.
Cautionary Note on Forward-Looking Statements|||This posting contains forward-looking statements for purposes of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. The posting may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Forward-looking statements include statements regarding intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things: the ability to initiate and progress clinical trials of allogeneic CAR T therapies, and the potential benefits of allogeneic CAR T therapy. Various factors may cause differences between Allogene’s expectations and actual results as discussed in greater detail in Allogene’s filings with the Securities and Exchange Commission (SEC), including without limitation in its Form 10-Q for the quarter ended June 30, 2019. Any forward-looking statements that are made in this posting speak only as of the date of this posting. Allogene assumes no obligation to update the forward-looking statements whether as a result of new information, future events or otherwise, after the date of this posting.