Novotech CRO Expands China Team as Biotech Demand for Clinical Trials Increases up to 79%
An increase in demand of up to 79% for clinical trials in China has prompted Novotech the Asia-Pacific CRO to rapidly expand the China team, appointing expert local clinical executives to their Shanghai and Hong Kong offices. The company is planning to expand their team by 30% over the next quarter.
 Dr John Moller, CEO, Novotech
Dr John Moller, CEO, NovotechNovotech China has seen considerable demand recently which is borne out by research from GlobalData:
A global migration of clinical research is occurring from high-income countries to low and middle-income countries with emerging economies. Over the period 2017 to 2018, for example, the number of clinical trial sites opened by biotech companies in Asia-Pacific increased by 35% compared to 8% in the rest of the world, with growth as high as 79% in China.
Novotech CEO Dr John Moller said China offers the largest population in the world, rapid economic growth, and an increasing willingness by government to invest in research and development.
Novotech’s 23 years of experience working in the region means we are the ideal CRO partner for USA biotechs wanting to tap the research expertise and opportunities that China offers.
There are over 22,000 active investigators in Greater China, with about 5,000 investigators with experience on at least 3 studies (source GlobalData).
Deloitte research also point to the benefits of new China National Medical Products Administration fast-track approval processes “and a potential local-study waiver for products targeting rare diseases or diseases with substantial unmet needs, leading to strong progression in new drugs approvals for both local and MNC companies”.
Dr Moller said the key to Novotech’s success across the region is a proven approach to delivering quality research that leverages local on the ground expertise.
“Our mission in China is to deliver the best research teams and investigators as well as the compliance and regulatory expertise that is demanded and expected by the NMPA and the FDA,” he said.
“We use the very latest in Oracle and Medidata technology, so seamlessly connect across all our offices and with global clinical partners.”
Checklist to see if the Asia-Pacific is right for your next study (takes 2 minutes) CLICK HERE
For RFP enquiries: Please fill out the form available at www.novotech-cro.com/contact-us-0
Watch Inside Novotech CRO
Novotech BIO US 19 Testimonial Highlight from Novotech on Vimeo.
 Yooni Kim Executive Director, Asia Operations, Novotech
Yooni Kim Executive Director, Asia Operations, NovotechQ&A with Yooni Kim Executive Director, Asia Operations
How does the US biotech sector perceive the Asia-Pacific region and how has it been evolving?
(Yooni Kim) The biotech sector has now a solid grasp of the benefits of running trials in Asia, which remains one of the fastest growing regions for biotech-sponsored trials. While Asia still offers complexities for the management of clinical trials, with differing cultures and regulatory requirements, partnering with a regional specialist CRO can assist in matching sponsor needs to country characteristics.Across Asia you get access to 1.4 billion often treatment-naive patients in easy and accessible urban areas.
Many changes have occurred recently in China. Could you tell us more about how it is affecting foreign biotechs?
(Yooni Kim) The NMPA (previously Chinese FDA) issued a series of regulatory reforms to address concerns around the promotion of R&D activity in China, especially from foreign companies. The main goals of the new reforms were mostly focused on improving the drug review process, shortening the Investigational New Drug (IND) and New Drug Application (NDA) review timelines and encouraging new drug innovation. Greater China has a pool of over 22,000 active investigators and a trial density that is much lower than in North America. As sponsors are facing increased competition in the US and Europe for investigators, sites, and patients, we see an increased demand from our clients to expand their clinical development over there.
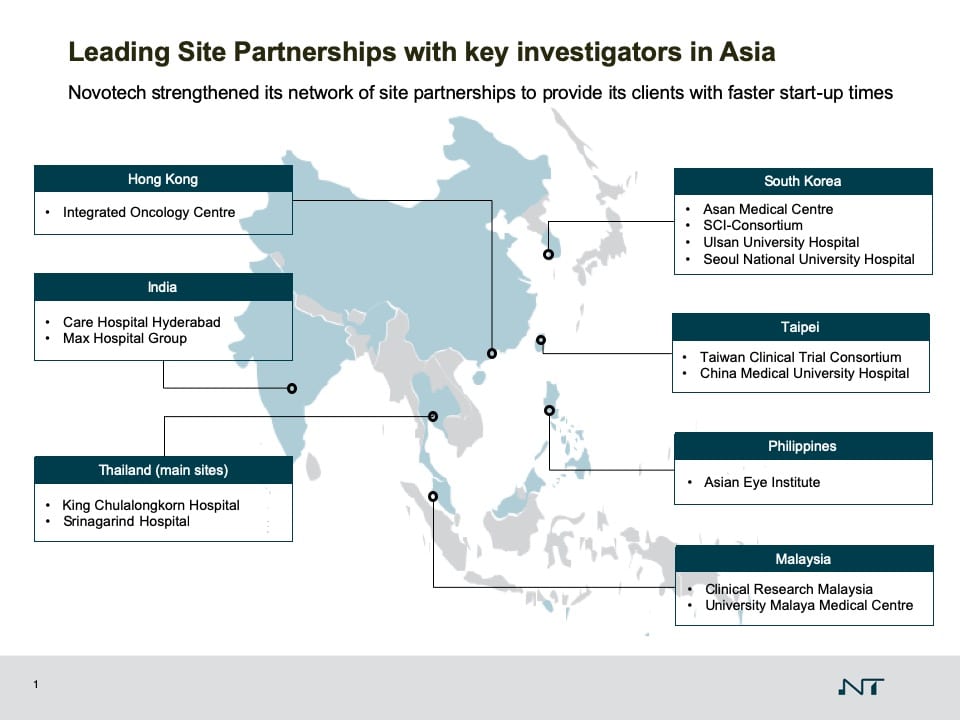
Novotech now has over 20 significant Partnerships with some of the leading medical institutions in the region. The Partnership Program is strategically designed to bring unparalleled access to quality investigators, KOLs, and up to 1.4 billion patients for its international biotech clients.
Click on the image to see the full-sized version
Key China Facts
- Relatively homogenous population (92% Han Chinese)
- Over 750 global companies have research centers in China
- Regulatory framework increasingly emulating the FDA structure
- Government pledge to dedicate USD18 billion to emerging biotechnologies in latest five year plan
- Largest clinical research market in Asia and 5th largest clinical research market in the world.
- The China National Medical Products Administration (previously CFDA) is the main regulatory body in China.
- Evaluation of biologics is stringent requiring assessment by and approved evaluation center.
- The average timeline for regulatory approval is now about 8 months compared with up to 24 months previously.
About Novotech CRO
Headquartered in Sydney, Australia Novotech is internationally recognized as the leading Asia-Pacific region’s full-service contract research organization (CRO). Novotech has been instrumental in the success of hundreds of Phase I – IV clinical trials in the Asia Pacific region.
Novotech has been based in the Asia Pacific region for more than 24 years and is well established with offices in 11 countries and more than 600 staff.
Novotech provides clinical development services across all clinical trial phases and therapeutic areas including: feasibility assessments; ethics committee and regulatory submissions, data management, statistical analysis, medical monitoring, safety services, central lab services, report write-up to ICH requirements, project and vendor management. Novotech’s strong Asia Pacific presence includes running clinical trials in all key regional markets. Novotech also has worldwide reach through the company’s network of strategic partners.