
Pioneering Click Chemistry in Humans
This article was published prior to the announcement of the 2022 Nobel Prize in Chemistry. Congratulations to Dr. Bertozzi, Dr. Meldal, and Dr. Sharpless.
Reimagining cancer treatments
Cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020, which is nearly one in six deaths. Recently, we have seen incredible advances in novel cancer therapies such as immune checkpoint inhibitors, cell therapies, and antibody-drug conjugates that have revamped cancer care and improved survival rates for patients.
Despite this significant progress in therapeutic targeting, why are we still seeing such a high mortality rate? The reason is that promising therapies are often limited by their therapeutic index, which is a measure of the effective dose of a drug, relative to its safety. If we could broaden the therapeutic indices of currently available medicines, it would revolutionize cancer treatments. We are still on the quest to find the ultimate cancer medicine – highly effective in several cancer types, safe, and precisely targeted to the tumor site.
The precision of click chemistry
A simple yet incredibly powerful scientific approach may be the key to unlocking the hidden potential of cancer therapies and improving their therapeutic index. This concept is called “click chemistry”, and it was first introduced in 2001 by Hartmuth Kolb, M.G. Finn, and Nobel prize winner Barry Sharpless.i It involves chemical reactions in which two molecules rapidly “click” with each other, ignoring their surroundings. When these types of reactions are conducted in a biological setting, they are termed bioorthogonal chemistry, as there is no interference from or to native biochemical processes.
“Bioorthogonal chemistry conducted inside a human body could open a doorway to improve the therapeutic index of chemotherapeutics and unlock new biological effects from cancer medicines” says Neal Devaraj, Professor of Chemistry and Biochemistry at UC San Diego and advisor for Shasqi.

All of the news, delivered with full-text to your inbox. For professionals discovering, developing, and marketing biopharmaceutical drugs.
Pioneering click chemistry in humans
Shasqi, a clinical-stage biotechnology company, has created the Click Activated Protodrugs Against Cancer (CAPAC®) platform, which uses click chemistry to localize and activate a ‘protodrug’ at the tumor site. Protodrugs are attenuated versions of powerful cancer therapies. When the protodrugs reach the tumor, their activation leads to the release of the full-strength payload (active drug) at the tumor site.
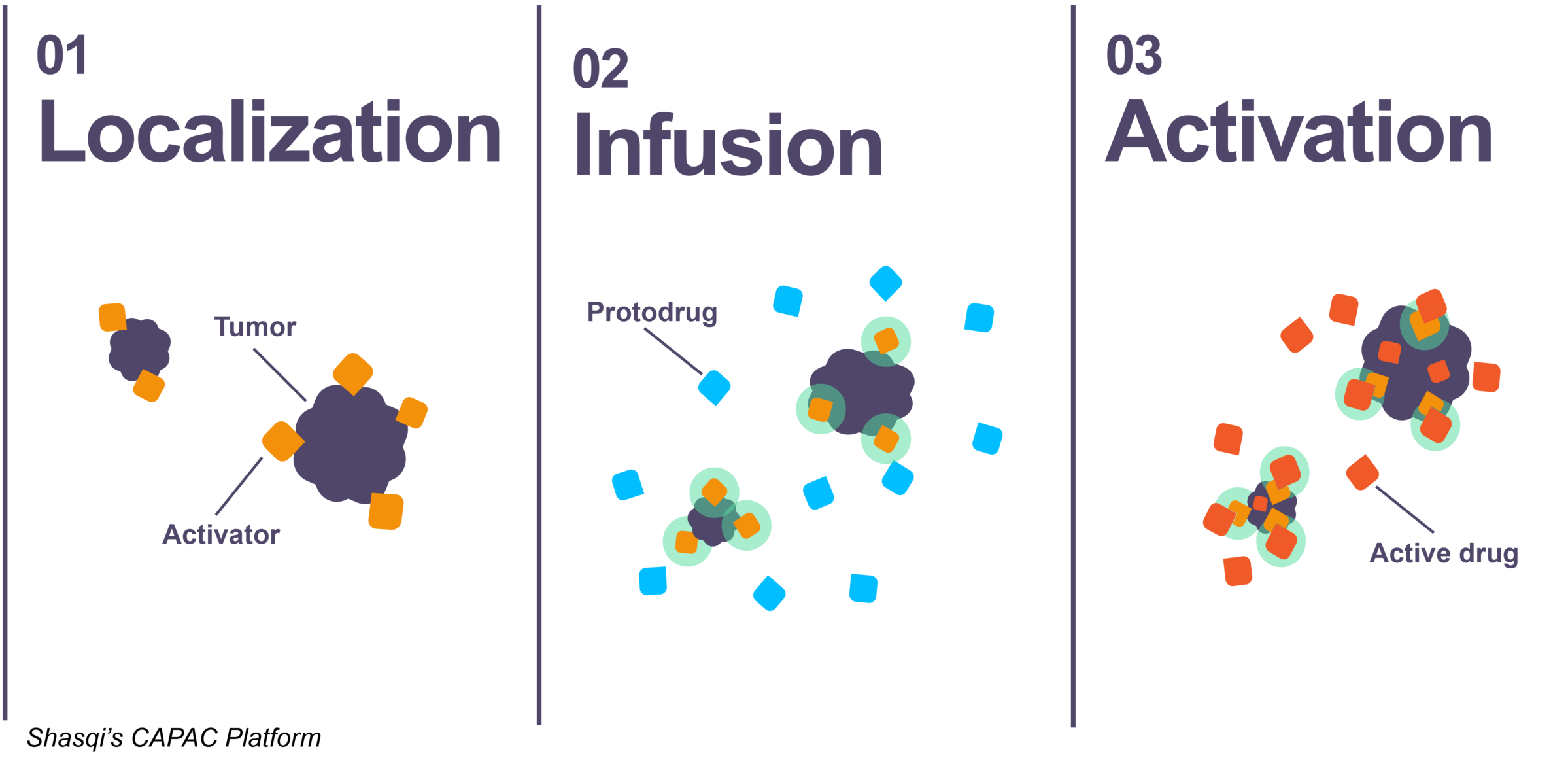
Shasqi’s first generation approach employs an intratumoral biopolymer injection to localize the protodrug activators at the tumor site. Shasqi’s lead asset, SQ3370, is based on a doxorubicin (Dox) protodrug and has established click chemistry in humans. SQ3370 is well-tolerated in patients in a phase 1 study. Despite dosing more than 10-times conventional Dox and up to 12 cycles, a maximally tolerated dose has not been identified to date.
SQ3370 unlocks the opportunity to exploit the full potential of the drug class. Novel immune regulatory effects, as described preclinically in 2021, were confirmed in humans. Increased cytotoxic T-cell activity observed at the higher dose levels of SQ3370 (> 6-times the conventional Dox dose) indicate immune activation, trending towards an increase in tumor cell death in heavily pretreated patients.
These findings establish exciting possibilities for SQ3370 and pave the way for advancing the program to Phase 2, which is currently enrolling patients. More importantly, they highlight how click chemistry in humans could one day truly transform cancer therapies and motivate the development of new protodrugs (e.g. auristatins, and taxanes) as well as new tumor-targeting agents.
Click chemistry and antibodies
Antibody drug conjugates (ADCs) enable the use of highly toxic and effective chemotherapeutic agents by linking tumor-targeting antibodies to potent payloads. ADCs have improved the lives of many cancer patients, but clinical applications remain limited due to narrow therapeutic indices and a limited set of suitable antigens that meet the criteria of ADCs.
By localizing click chemistry activators to the tumor with antibodies, protodrugs can be activated and payloads released at the tumor site. The CAPAC platform decouples the tumor-targeting component from the payload, spearheading the pursuit of non-internalizing antigens.
This feature expands potential target antigens to those that do not reside on the surface of tumor cells (e.g., in the tumor microenvironment). These antigens represent exciting new targets that are uniquely suited for click chemistry in humans. They hold great promise to expand the indications suitable for antibody-targeted therapies, especially with regards to solid tumors.
In addition, separating the tumor-targeting component from the payload enables unique therapeutic combinations. A single targeting agent can be used with two or more protodrugs, each exploiting a different mechanism of action. These potential regimens are currently inaccessible due to overlapping toxicities.
Disrupting cancer therapy as we know it
Improving the therapeutic index of cancer medicines is one of the biggest challenges we face today. Shasqi has unlocked click chemistry in humans and is poised to apply the technology to create unprecedented opportunities to explore curative options.
A key to accelerating the rate of progress against cancer lies in collaborating to activate the untapped potential of powerful cancer therapies by leveraging the precision of click chemistry.
“Bioorthogonal chemistry has the potential to improve the utility and outcomes for many anti-cancer treatment modalities. We see a future where combinations of therapies can be activated at desired areas of the body, resulting in much greater safety and efficacy than we have today” notes Carolyn Bertozzi, Professor of Chemistry at Stanford University and advisor for Shasqi.
References:
[i]Kolb, Finn, Sharpless, Angew. Chem. Int. Ed. 2001, 40, 2004-2021.