Putting Data to Work in a Pandemic
Medidata’s Intelligent Trials is helping clinical trials sponsors dig out of a pandemic-led slump with powerful data resources and predictive analysis
July marked a turning point in the COVID-19 pandemic for oncology clinical trials. With coronavirus safety protocols better established in clinical settings, cancer patients who had stayed away for months began to return to trials. New patients entering studies increased 20 percent versus 2019 baseline levels. Just three months earlier, in April, at the beginning of the pandemic, new patient recruits plunged 40 percent versus the previous year.
The July turnaround was good news for oncology researchers, pharma companies and, of course, cancer patients. It meant that many oncology clinical trial sponsors could begin to reboot their trials and get back to work again on designing and testing life-saving cancer drugs. But sponsors in the oncology field, and every other therapeutic category, still need a lot of help.
Even before the pandemic hit, clinical trials were beset by high failure rates due to poor site performance, recruitment and data quality issues. Nearly 90% of all trials were unable to enroll patients within target timeframes, while each day of delay in a drug’s time to market costs sponsors an estimated $600,000 to $8 million. The COVID-19 pandemic only compounded problems for study starts, enrollment, and patient completion of visits. As the business begins to dig itself out of a pandemic-led slump, sponsors need to know, now more than ever, where to find high performing sites and how to ensure that their enrollment and data collection efforts are on target.
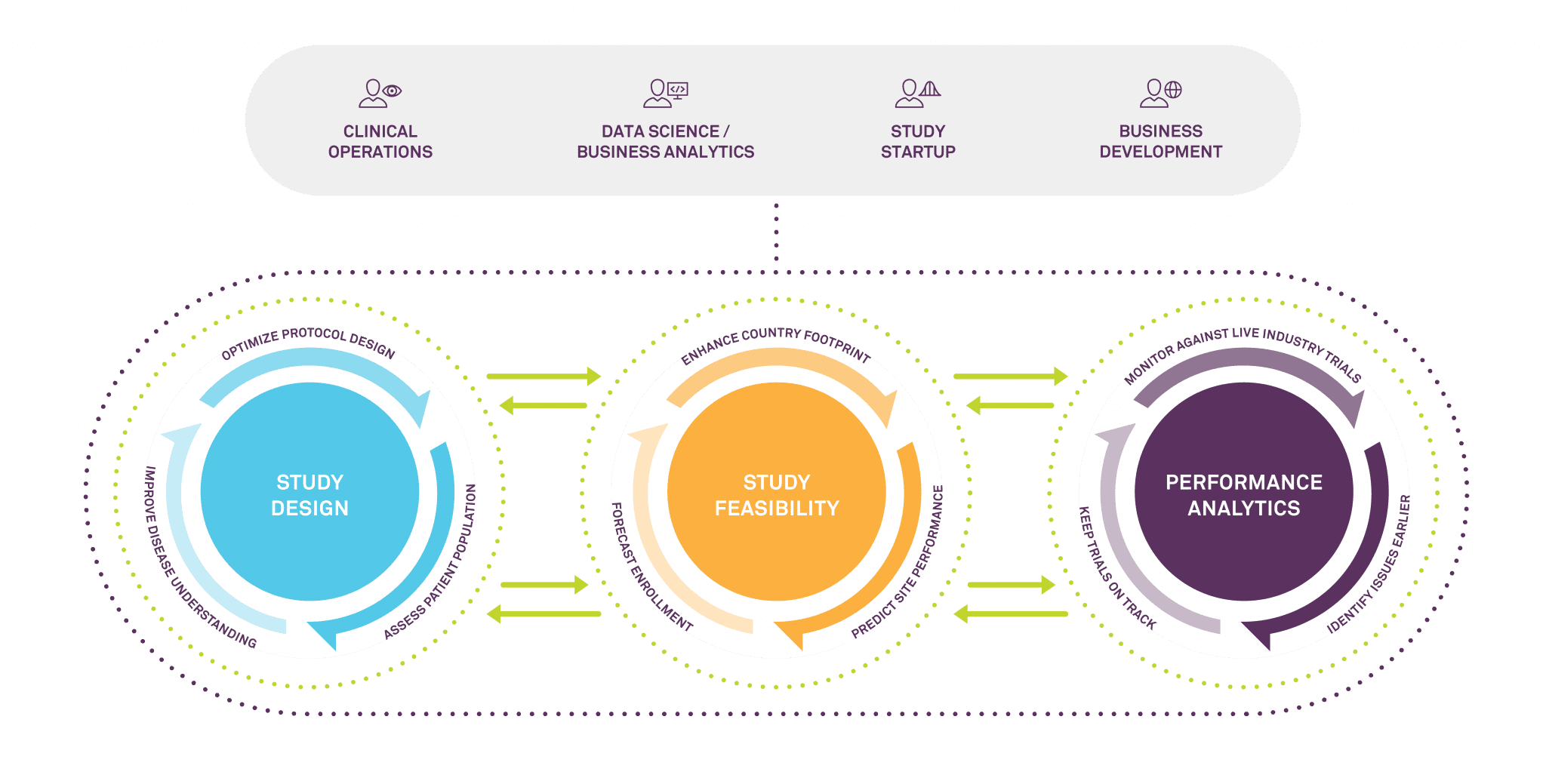
Medidata’s Intelligent Trials program is designed to provide answers to these questions. The analytics platform aims to improve the speed, success, and quality of trials through better study design, enhanced country and site selection, enrollment predictions, and real-time study tracking against other similar industry trials. Most importantly, it is built on the most comprehensive, fine grained and enriched repository of clinical trial data in the business. A long history of trusted relationships with the industry’s largest players means Medidata has access not only to unparalleled historical data resources, but also extensive current insights. Its datasets cover 20,000 plus historical trials and 22,000 healthcare facilities with associated investigators across 94 countries, as well as 6,000 live trials happening in real time.
The real-time data Medidata collects and curates is especially crucial in these extraordinary times. Just as Medidata can help its customers tell which sites did well before the pandemic, it can see which sites are coming back up to speed quickly as the pandemic progresses. It can help to stratify the data to look at how patient accrual differs by country and by therapeutic or disease area. And while the pandemic itself is unprecedented, even now our predictive modelers are looking at how we can quantify the specific impact of the pandemic on a given site’s operational performance over time.
Predicting the Future
Medidata got a jump start in data analytics almost a decade ago. In 2010, the company’s executives had the foresight to reach out to the biggest names in the pharma business to invite them to share data in a coordinated way. In a spirit of cooperation, the companies agreed to let Medidata pool their data to provide analysis that would improve clinical trials operations for everyone, while protecting the data privacy of individual patients and research organizations. No one else in the industry has access to clinical and clinical trial operational data all in one place from hundreds of sponsors and multiple clinical research organizations.
It’s not just the quantity of data that makes Medidata’s platform exceptional. It’s also the level of detail that it can offer. While publicly available clinical trial datasets exist, these do not provide the fine grain of information that Medidata’s datasets do. Its platform has information from the actual execution of each study, for example. That data can be combined to give deep insight into performance of different diseases, countries and sites.
Medidata’s data is also enriched through a two-step process that is unique in the business. Other companies use either algorithms or manual curation to structure their data, but Medidata’s approach is a hybrid of the two. We let the machines do what they do best: learn from the data and apply systems of classification. Then we bring subject matter experts into the loop to adjudicate the findings and complete structuring the data before it is used in analysis. We also leverage publicly available data from all over the world including trial registries and publications.
Putting the Data to Work
All of the metrics and insights from the clinical trials data we collect can then be fed back into the study design process, enhancing recruitment and enrollment as well as timing and cost. We use both historical and real time performance data to train predictive models, to anticipate future performance, to determine the likelihood that a particular site will successfully enroll for a particular kind of trial. Study site performance depends on many things, of course, such as how interesting the study drug is and how congested the geographical area is in terms of similar studies. Today, it also depends on COVID-19 rates in a particular geography and the COVID-19 risk factors of the particular patient population that is targeted.
Our Intelligent Trials predictive models can take into account hundreds of features, including a trial’s country footprint, as well as the performance, congestion, quality and capacity of individual sites and collections of study sites on different timelines. Rather than waiting for problems to arise, you can generate forward-looking insights that accelerate patient enrollment based on historic performance, predictive models, industry trends, and changing conditions in real time. And you always have access to the input of our team of experts to help you evaluate these insights, including not just data scientists and technologists, but ex-regulatory officials and investigators.
As an example of how this can work, Intelligent Trials recently helped a large clinical trials sponsor accelerate the completion of an underperforming phase III trial for an experimental treatment that was facing slowed enrollment. Medidata performed a study design complexity analysis and site-by-site analysis to help the sponsor identify those sites requiring intervention and select new sites that had potential to speed up timelines. Ultimately, the trial was accelerated by at least 6 months versus anticipated timelines. That helped advance time to market and resulted in a huge cost savings for the sponsor.
Medidata has created its Intelligent Trials insights to empower sponsors to build the best possible clinical trials in the most challenging of environments. Our mission is aligned with theirs: to help bring life saving treatments to patients who need them as quickly as possible, in spite of the pandemic. We want to help build a better world.