
Solving the challenge of nucleic acid delivery in RNA medicines
Cell and Gene Therapy is shaping up to be the next major growth area for the Pharmaceutical Industry. The US Food and Drug Administration plans to hire 50 new clinical reviewers to handle the 200 plus IND applications per year the agency is expecting to receive by 2020 for new therapies to enter clinical trials. In a statement, FDA Commissioner Scott Gottlieb and the director of the Center for Biologics Evaluation and Research, Peter Marks predict that the agency may be approving 10–20 new cell and gene therapy products a year by 2025.¹
The path however is not without difficulties. Unmodified nucleic acids can cause a strong immune response and are quickly degraded by enzymes in the bloodstream and the gut. Therefore, RNA medicines must be delivered to target cells using a vector. Viral vectors and lipid-mediated delivery systems are the two main approaches used to deliver intact, bioactive RNA to target cells in therapeutic development.
These delivery systems can create their own unwanted side effects. Viral vectors and existing lipid vector technology can cause liver damage and activate an adverse immune response in human patients. Viral vectors may cause accidental mutations in host DNA, which can lead to cancer. Patients treated with viral vectors can also develop antibodies against these vectors that make the treatment less effective over time. There is clearly a need for improved delivery vehicles before RNA therapies can become mainstream.
Arcturus’ has developed a proprietary lipid nanoparticle delivery system which enables multiple nucleic medicines with a focus on large RNA. The LUNAR delivery system effectively addresses the issues that plague existing viral and lipid vector delivery systems. As well as its own in-house development programs Arcturus is applying the LUNAR platform through established partnerships with a number of prominent Pharmaceutical companies including Janssen, Takeda, and UltraGenyx amongst others.
LUNAR®
The LUNAR technology can effectively and safely deliver large RNA molecules into targeted cells within a patient’s body. LUNAR® lipid-mediated delivery technology includes a diverse, growing library of over 160 proprietary lipids that were rationally designed to be versatile, maximizing efficacy and increasing tolerability of a diverse selection of nucleic acids, target cell types and routes of administration. A key feature of LUNAR® lipids is their biodegradability, decreasing the undesired effects caused by lipid accumulation that are associated with existing lipid-mediated platforms, resulting in tolerability that can decrease efficacy of the therapy or even cause treatment to be halted.
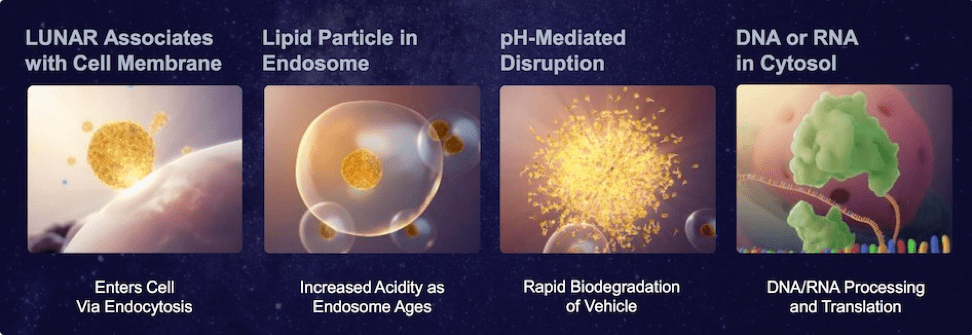
LUNAR® technology can be applied to all types of RNA medicine, but it has most commercial opportunity and value in delivering large RNA. These large RNA molecules show the greatest immediate promise for RNA gene therapy, but because of their size and poor stability they have traditionally been the hardest to administer therapeutically. The LUNAR platform is also able to deliver other smaller therapeutic RNA molecules including antisense RNA, RNAi, siRNA, or microRNA. Companies using small RN/small molecules can also benefit.
Benefits of LUNAR®
- LUNAR® delivery technology can be used with multiple types of nucleic acid therapeutics.
- LUNAR® can be administered by multiple routes (IV, IM, nebulized, subretinal, and intravitreal).
- LUNAR® can target multiple different clinically important cells including stellate cells, hepatocytes, lung epithelial cells, eye cells, and myocytes.
- LUNAR® can deliver mixtures of different RNAs as one drug product.
- Preclinical studies show that LUNAR compounds, unlike other LNP vectors, have a wide therapeutic index.
- LUNAR® lipids are optimized for high RNA and DNA encapsulation efficiency, which is important for the cost of manufacturing.
Arcturus’ Pipeline of mRNA Medicines
Arcturus aims to leverage the proprietary and licensed intellectual property relating to LUNAR® and the Nucleic acid technologies to develop a pipeline of mRNA therapeutics for infectious rare diseases and rare genetic disorders with significant unmet medical needs. The two flagship programs; LUNAR®-OTC and LUNAR®-CF, are on track for IND submission in 1Q2020 and 2H2020 respectively.
LUNAR®-OTC
OTC deficiency is caused by mutations in the OTC gene which leads to a non-functional or deficient OTC enzyme. OTCD is the most common urea cycle disorder. Urea cycle disorders are a group of inherited metabolic disorders that make it difficult for afflicted patients to remove toxic waste products as proteins are digested.
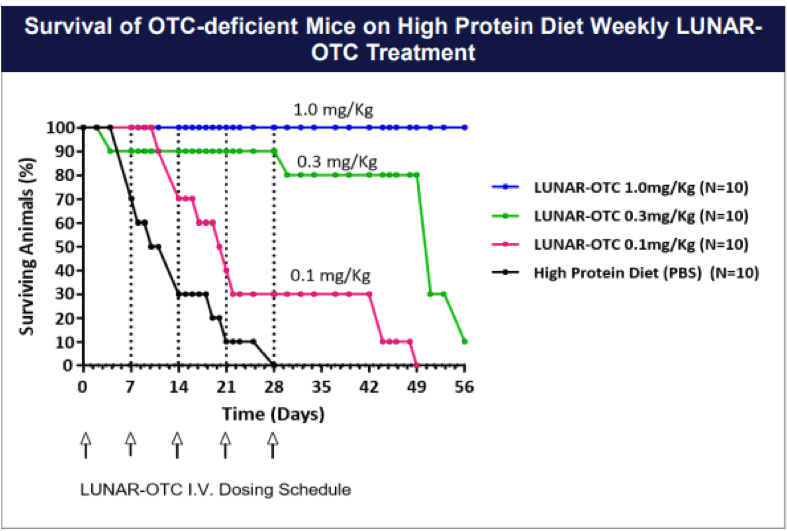
Preclinical proof-of-concept studies have shown that LUNAR®-delivered human OTC mRNA reduces urinary orotic acid levels in a well-established mouse model of OTC deficiency: OTC-spf ash mice. These mice have elevated urinary orotic acid. Because they have a small amount of residual OTC enzyme activity, they are not hyperammonemic unless challenged with a high protein diet through inhibition of the residual OTC enzyme activity. OTC-spf ash mice were treated with induced hyperammonemia resulting from a high protein diet, with one intravenous dose of LUNAR®-encapsulated human OTC mRNA (candidate mRNA sequences tested at a low, middle, and high dose level)
A LUNAR®-encapsulated Luciferase mRNA was included as a control. As shown in the figure below, this single treatment significantly reduced urinary orotic acid levels for at least seven days post-treatment (n=4-6 animals per group).
LUNAR®-CF
The LUNAR®-CF program addresses cystic fibrosis, a progressive lung disease caused by mutations in the CFTR gene.
Arcturus uses LUNAR® platform to deliver optimized CFTR mRNA into airway epithelial cells. This allows airway cells to produce functional CFTR protein using their native translational machinery and protein trafficking pathways. This approach has the potential to treat the underlying defect that causes CF (dysfunctional or absent CFTR protein) in all such patients, regardless of mutation type. The potential has been recognized by Cystic Fibrosis Foundation Therapeutics, Inc. (CFFT), with whom Arcturus has partnered with to develop this important therapy.
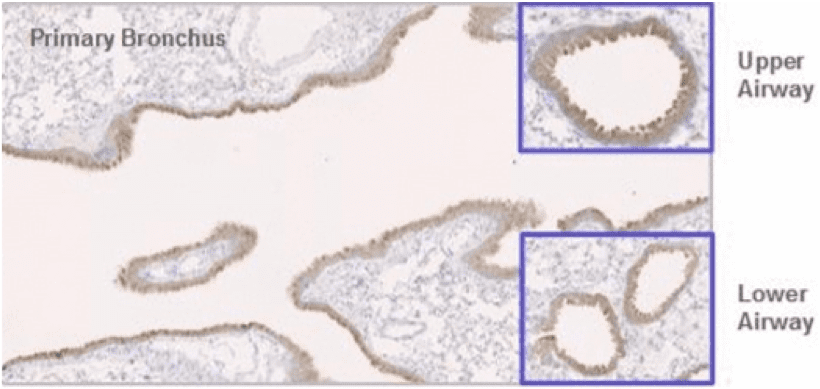
Arcturus had completed preclinical proof-of-concept studies, demonstrating that LUNAR® efficiently delivers a functional reporter mRNA efficiently into mouse lung epithelial cells in vivo (figure below). Six hours following intratracheal delivery of 0.4 mg/kg of the LUNAR®-encapsulated reporter green fluorescent protein (GFP) mRNA, GFP protein expression (shown in brown) was observed in mouse lung epithelial cells of the primary bronchus and in bronchioles, important lung structures, located in the upper and lower airways.
Arcturus continues to invest in the LUNAR® lipid-mediated delivery of mRNA (encoding CRISPR, TALEN, zinc finger proteins, and meganucleases), siRNA, DNA, microRNA, and antisense oligonucleotide technology platforms to improve their efficacy and safety profile, further expanding their applications. These investments have led to key innovations ensuring optimal characteristics of LUNAR® formulated drug products are attained, and this sets Arcturus apart from other nucleic acid therapeutics and lipid-mediated delivery platforms.
For more information on the LUNAR platform, contact Neda Safarzadeh, Director, Head of Investor Relations/PR/Marketing at 858.357.7894, or email at neda@arcturusrx.com.
References
- Cross R. FDA prepares for huge growth in cell and gene therapy. Chemical and Engineering News. January 16, 2019 | VOL 97, ISSUE 3