Taking Action to Ensure Clinical Trial Progress During COVID-19
Why does it take so long to get new vaccines and medicines to market?
It is not a new question. But as governments, communities and medical professionals around the world focus their energy and resources on containing the COVID-19 pandemic, it’s being asked with increasing urgency. It deserves – in fact, it requires – our collective attention and effort.
The good news amidst so many troubling headlines is that the technologies we need to improve patient access and experience are here now. The smart application of those technologies can overcome barriers to trial execution and improve data sharing and process efficiency across organizations. Most urgently, those technologies can be used immediately to ensure progress for thousands of clinical trials in an environment where patients are expected to stay at home.
Decentralized and hybrid trials – the time has come
At a recent congressional hearing, NIAID director Anthony Fauci gave a frank assessment about the shortcomings of the U.S. system for coronavirus testing: It’s “not really geared to what we need right now.”
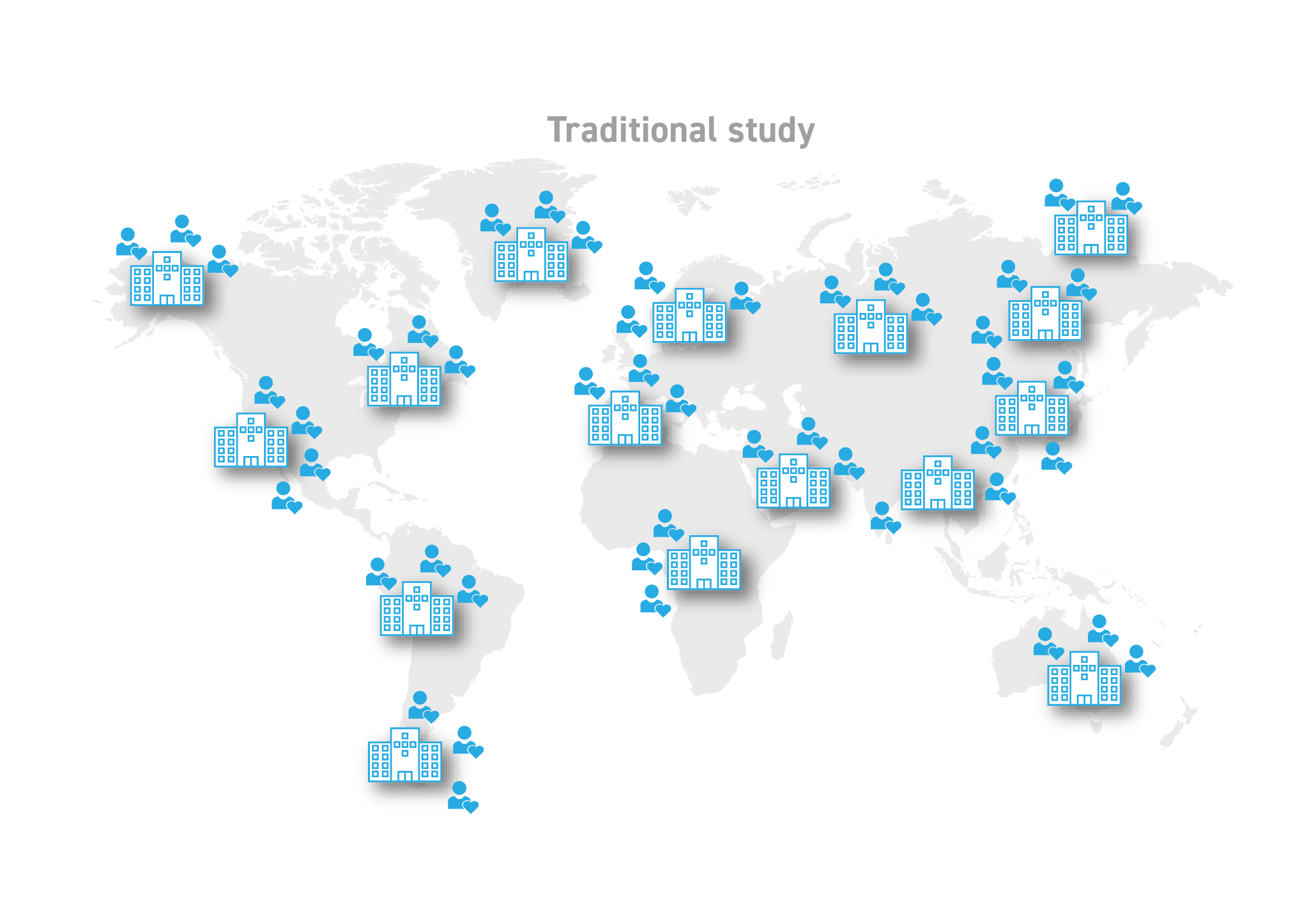
The same can be said for our traditional clinical trial model. Limiting trials to a handful or two of physical sites inherently limits patient access. Restricting interaction to in-person visits is not only grossly inefficient in many cases, but it also limits data frequency and quality. And during a crisis like the COVID-19 pandemic, it’s simply infeasible.
The traditional trial model does not put patient needs first.
Click on the image to see the full-sized version
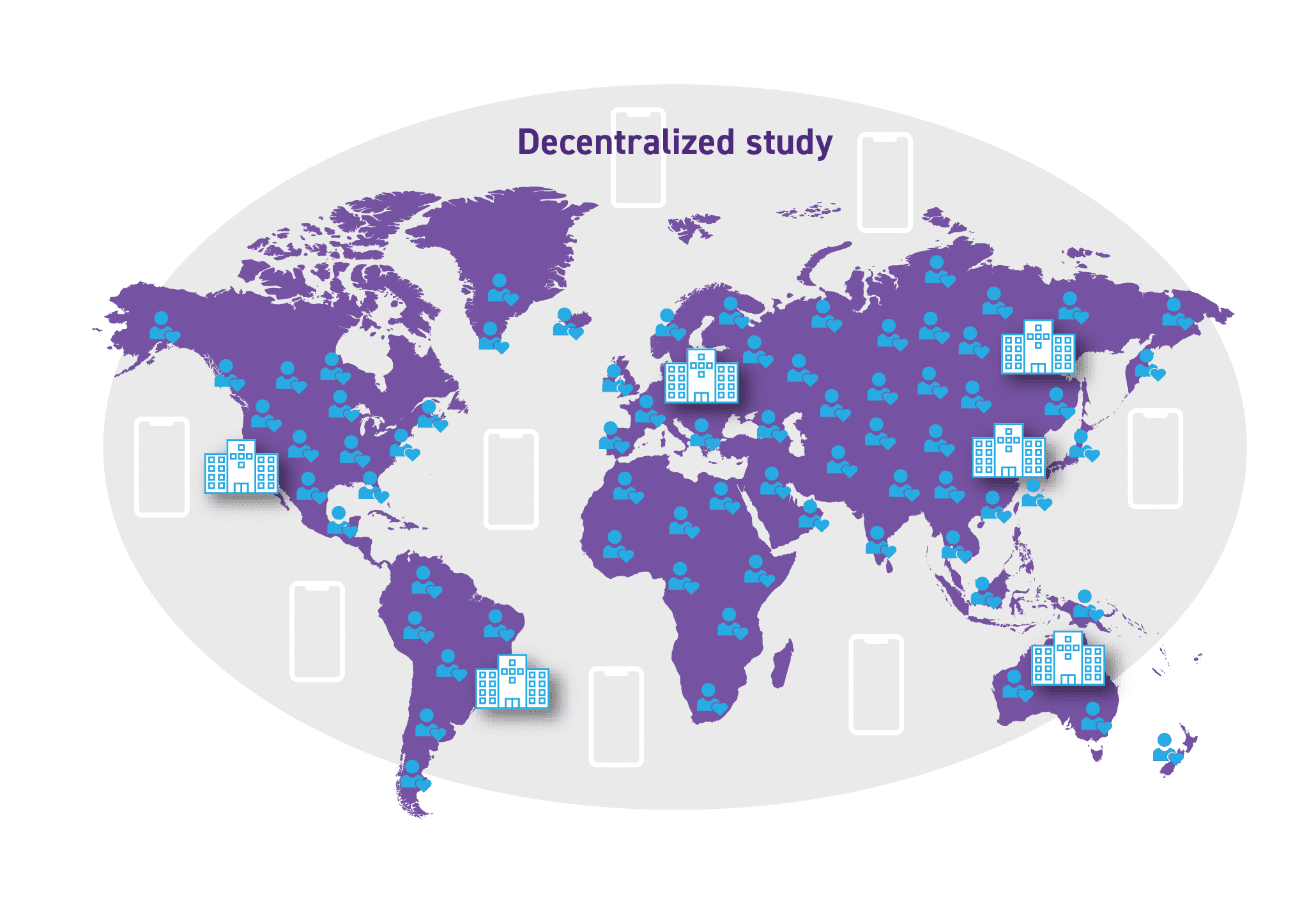
By contrast, decentralized clinical trials look more compelling than ever. The Clinical Trials Transformation Initiative (CTTI) defines decentralized trials as those trials executed through telemedicine and mobile/local healthcare providers, using procedures that vary from the traditional clinical trial model. In layperson terms, the trial is conducted remotely with the participant remaining at home.
The industry has been looking to decentralize trials for years. Now, as health authorities worldwide struggle to contain the COVID-19 outbreak, there is a renewed push to rapidly implement remote healthcare delivery capabilities.
There are currently more than 55,000 interventional clinical trials actively enrolling and providing care for participants worldwide. In light of the current outbreak, it is critical that we continue to deliver high-quality healthcare to research participants, while also continuing to advance clinical drug development programs.
Decentralized trials are largely “geared” for exactly this type of situation. Of the 55,000 trials in flight, some are good candidates for a fully decentralized model — while many others can be managed in a hybrid model. Patients can be recruited and consented remotely. Physician “visits” can be conducted remotely via telemedicine. Data can be captured remotely (and frequently) via medical devices and mobile technology.
The Decentralized Trial model provides global patient access with increased data frequency and quality.
Click on the image to see the full-sized version
All of this expands our ability to conduct research by “untethering” it from physical sites — critical when people around the world are being told to “stay home” due to COVID-19. Going digital (where possible) reduces patient risk of pathogen exposure, while potentially accelerating drug and vaccine development. That may require some changes to study design or regulatory approvals, but we’re finding it’s a priority for many clinical leaders right now — especially those that need to ensure productive trial progress and avoid significant loss of patient participation.
Working together to accelerate COVID-19 research
In addition to accelerating decentralized trial adoption, there’s an urgent need for creative and collaborative solutions for COVID-19 research. To wit, several companies (including Medable) recently joined forces to streamline the path for diagnostics, health monitoring and clinical trials for COVID-19. The ACCESS initiative—short for American COVID-19 Collaborative Enabling Seamless Science—provides a mobile consumer application and secure infrastructure to quickly connect health researchers and clinical trial teams securely with up to millions of home-bound individuals in the United States.
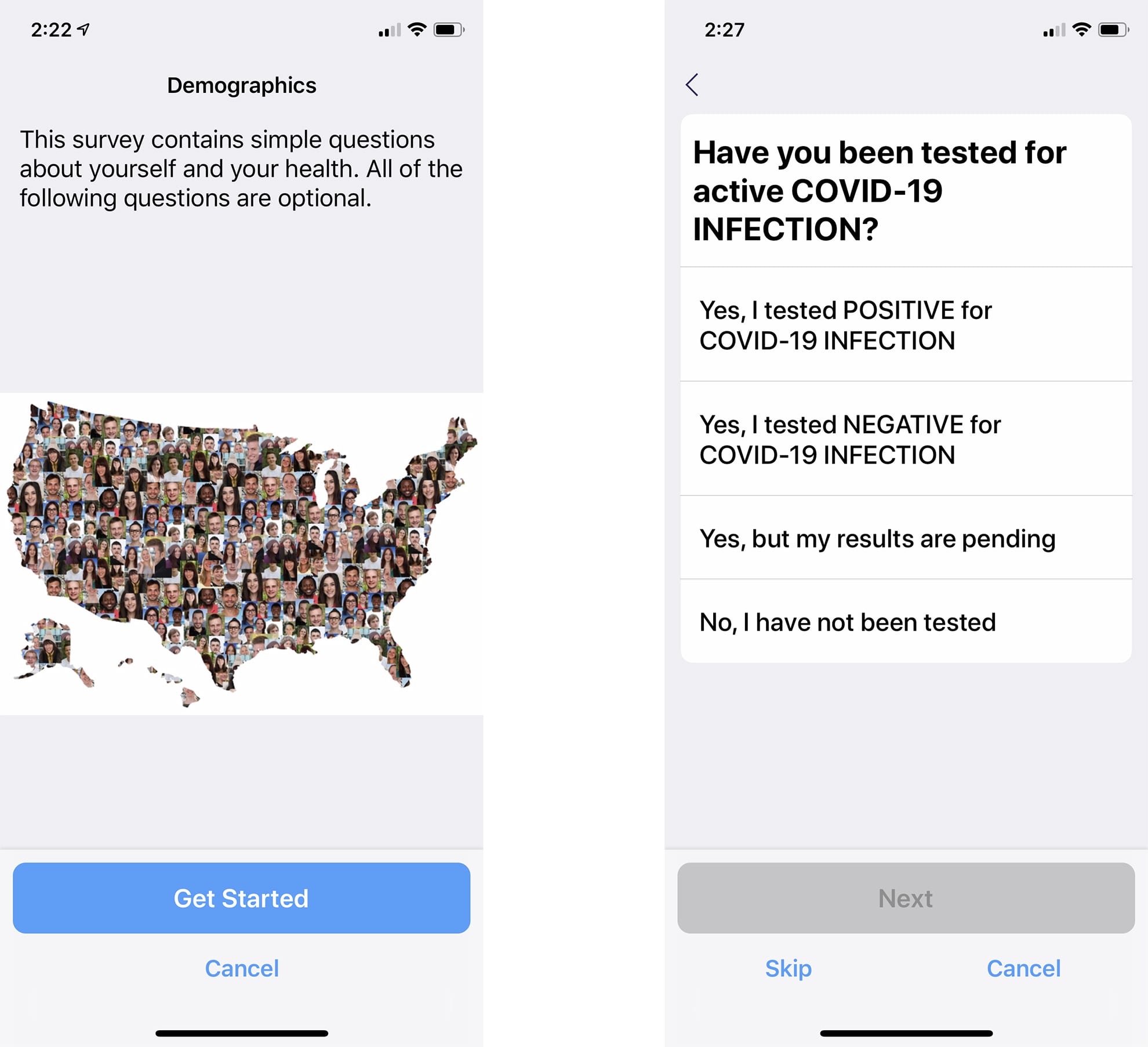
ACCESS makes it easy for individuals to contribute specific information about their COVID-19 experience, combine it with health records and data from wearable devices, and opt in to participate in current and future studies for diagnostics, treatments and vaccines. The data that people share can be quickly and anonymously matched to research studies, providing researchers with a foundational framework for dynamic research at scale.
The mobile app makes it easy for individuals to provide health data and be identified for applicable studies.
Click on the image to see the full-sized version
ACCESS is a collaborative effort led by Medable, together with technology, healthcare and life sciences companies including BioIntelliSense, Datavant, Parexel, PWNHealth and the American Heart Association’s Center for Health Technology and Innovation. ACCESS takes full advantage of mobile and digital health technologies to facilitate at-home research, clinical trial access, and population-based long-term outcome studies. The infrastructure combines medical-grade wearable sensors, patient-reported data and outcomes, historical health data and health record aggregation. Participants opt in at every stage, so they maintain control over their personal health data—and decide how they want to engage in potential studies.
We believe ACCESS will enable us all to accelerate diagnostic testing and clinical trials—and advance important monitoring and immunity research—so that we can conquer COVID-19 with effective prevention and intervention strategies. For more information on ACCESS, go to https://access.medable.com/.
Let’s mobilize
With all of this in mind, there is an immediate opportunity for decentralized trials and creative digital solutions to improve patient access, experience and outcomes. Let’s work together as a community to drive forward faster, whether it’s streamlining trials for COVID-19 vaccines, reducing timelines for other therapies, or initiating new trials to address the 7,000 rare diseases that have no therapies on the market.
The COVID-19 outbreak has made it crystal clear: Decentralized trials are no longer a nice-to-have. Reducing trial timelines has to be a long-term industry imperative, and digital technology can help valuable research continue to move forward while keeping participants safe. Let’s start now, and let’s move faster together.
Alison Holland is the head of decentralized and remote trials at Medable. Ali has more than 30 years of clinical trial experience, most recently as Global VP & General Manager for General Medicine at Covance, a leading global clinical research organization. Ali has managed more than 300 clinical trials, working successfully with biotech organizations as well as global pharma on some of their most critical initiatives.