
The dawn of venture capital-backed cryo-EM startups
Last month, Septerna, Inc., became the latest in a recent string of new biotechs to launch by building on cryo-electron microscopy (cryo-EM) and other transformative platform technologies, to deliver novel therapeutics for new and well-validated drug targets alike. With financing led by Third Rock Ventures, Septerna, Inc., closed a $100M Series A financing round to leverage its proprietary Native Complex Platform for cryo-EM enabled structure-based drug design, to target native G protein-coupled receptors (GPCRs) with novel small molecule medicines, covering a wide range of therapeutic areas.
Like Septerna, Inc., other biotechnology companies, such as MOMA Therapeutics, Generate Biomedicines, and Gandeeva Therapeutics, Inc., use cryo-EM as an essential innovation platform to drive both target validation and novel therapeutic modalities. Cryo-EM also contributes to the success of related platform companies, like Sosei Heptares, ConfometRx Inc., and Astex Pharmaceuticals Inc., as well as numerous big companies that have integrated cryo-EM into their workflows.1
How the cryo-EM revolution propels structure-based drug design for difficult targets
The track record of GPCRs as the most successful drug target class is well known. Despite that success, the full potential of this target class remains untapped. Researchers from Sosei Heptares, a leading GPCR structure-based drug design company, suggest that less than 13% of the potentially useful therapeutic modalities pertinent to GPCRs have been successful to date.2 The under-exploitation of GPCRs is in no small part because targeting GPCRs often calls for highly receptor-selective and tissue-specific drugs. In a recent scientific article, researchers from Sosei Heptares described how structure-based drug design could be used to discover subtype-specific muscarinic receptor agonists that primarily act in the brain.3 A press release later announced a $2.6 billion deal with Neurocrine Biosciences to clinically advance their selective muscarinic receptor agonist portfolio for the treatment of neuropsychiatric disorders. In other words, structure-based drug design enables discovery of highly valuable drugs for historically hard-to-drug targets.
Septerna, Inc.’s scientific founders, Robert Lefkowitz, MD, Arthur Christopolous, PhD, and Patrick Sexton, PhD, DSC, are pioneers of GPCR biology, as well as world leaders in developing GPCR-tailored workflows to maximize cryo-EM output and impact. While not restricted to GPCRs, cryo-EM has rapidly advanced since Drs. Jacques Dubochet, Joachim Frank, and Richard Henderson were awarded the 2017 Nobel Prize in Chemistry for its development. The technique has become the gold standard method for structure determination of active GPCR complexes, and it is now poised to do the same for inactive-state GPCRs, as well.
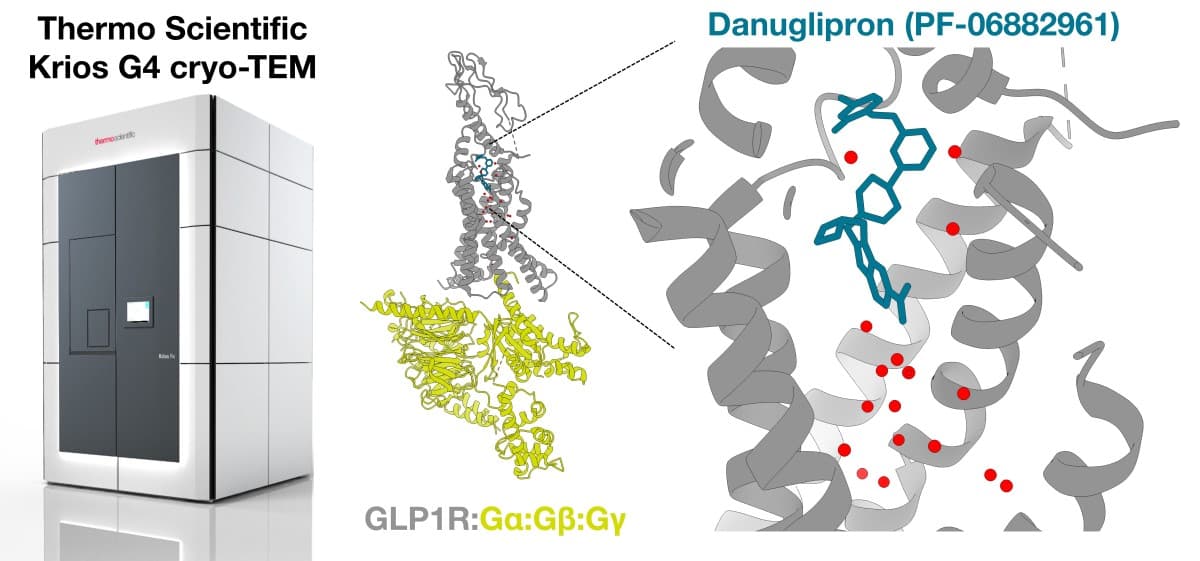
Figure 1. Cryo-transmission electron microscopes (cryo-TEMs), like the Thermo Scientific™ Krios™ G4 Cryo-TEM, bring the benefits of structure-based drug design to challenging targets like glucagon-like peptide 1 receptor (GLP1R). High-resolution cryo-EM model visualizing in blue: Pfizer’s clinical-stage GLP1R agonist danuglipron/PF-06882961 and red: surrounding water network.
A glimpse into the capabilities of cryo-EM structure-based drug design and the Septerna, Inc. team’s intimate understanding of GPCRs is provided through visualizing the mechanism by which allosteric modulation of adenosine A1 receptor (A1R) can mediate analgesia in a context-specific manner.4 Allosteric modulators are ligands that bind away from the binding site of native GPCR ligands and to pockets that can subtly tweak a receptor’s function. This is akin to using a dimmer switch rather than a strict on/off switch and provides more control over side-effects. Furthermore, allosteric binding sites are less conserved and better suited for designing receptor subtype-specific compounds. Drs. Christopolous and Sexton used cryo-EM to discover an allosteric binding pocket of A1R and show that it could be exploited to design non-opioid analgesics that exert their function in the spinal cord, providing significant promise for the treatment of neuropathic pain.
From cryo-EM-aided to cryo-EM-driven drug design for chemical diversity and speed
For drug targets with a complex biology, finding the narrow path to drug design requires innovative chemistry and reliable assays that are predictive for the therapeutic hypothesis. Cryo-EM platform companies offer the promise of unprecedented access to drug-like chemical space and compressed drug discovery timelines by using in silico methods as the driving force of compound design.
Conventionally, pharmaceutical companies synthesize thousands of compounds per drug discovery program, testing their functions in biological assays. However, developing appropriate assays can be difficult, lengthy, and expensive. While structure-based drug design is known to aid the efficiency of such design-make-test-analyze (DMTA) cycles, it does not traditionally drive small molecule discovery.5
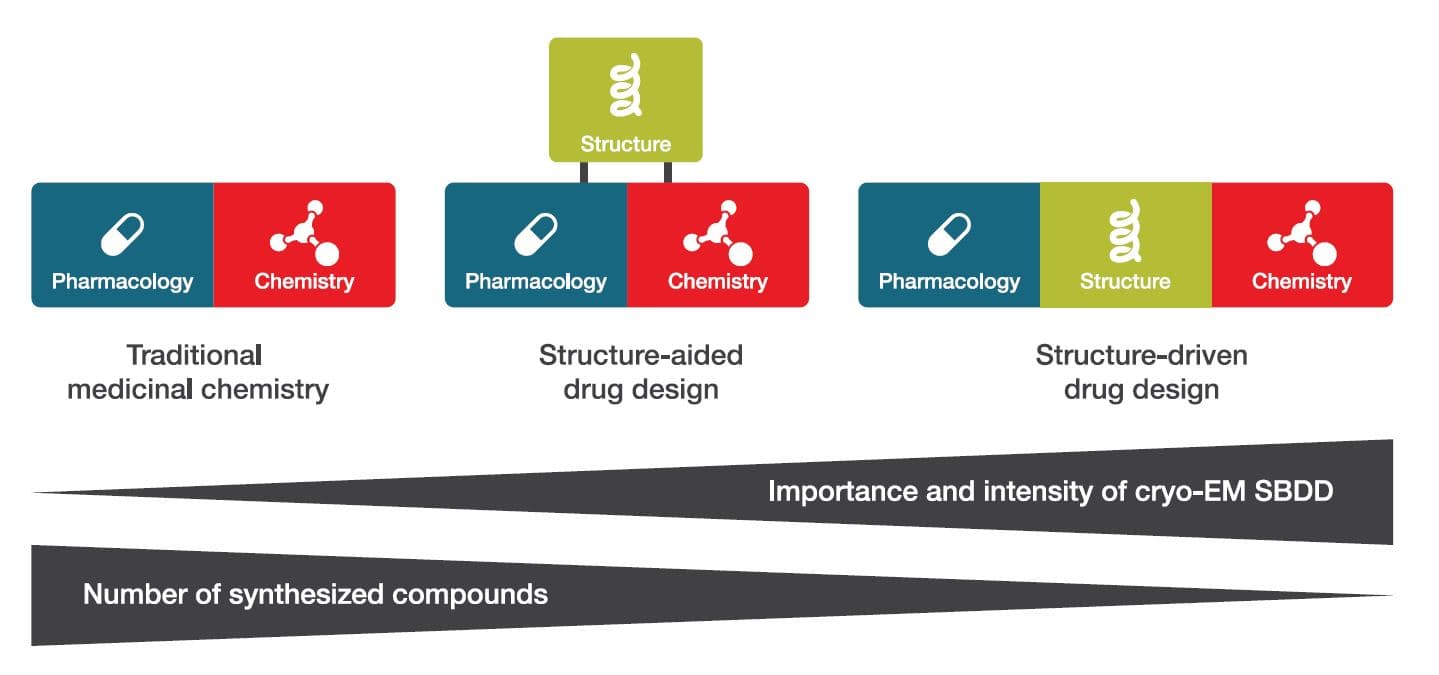
Figure 2. The role of structure in drug design is rapidly evolving from a supporting role to the driving force.
In drug design that is driven by cryo-EM structure-based drug design, high-resolution structures of target–ligand complexes can provide readouts to fine-tune pharmacological properties and reduce reliance on biological assays. A noteworthy example is the analysis of GLP1R structures, which is a prominent GPCR target for diabetes and weight loss. For GLP1R, pharmacological endpoints correlate with molecular determinants such as critical water networks or changes in receptor dynamics that can be visualized with cryo-EM.6,7 Furthermore, cryo-EM structure-based drug design is particularly powerful when integrated with computational methods that can evaluate billions of drug-like compounds per week.5 Dramatic advances in physics-based methods and in the capabilities of artificial intelligence give cryo-EM structure-based drug design the power to achieve highly optimized drug candidates with far fewer DMTA cycles. In summation, cryo-EM structure-based drug design enables companies to discover better drugs for important diseases in less time.
Thermo Scientific™ cryo-EM SBDD platforms and eco-system of drug target excellence
Thermo Fisher Scientific is the world leader in cryo-EM instrumentation and is unrivaled in unlocking the full potential of cryo-EM structure-based drug design for next-generation small molecule, protein degraders, biologics, cell, and gene therapies. Thermo Scientific cryo-TEMs provide the highest resolution, structure throughput, and ease-of-use.8 The newest instruments implement artificial intelligence to deliver unparalleled automation that provides a strong competitive edge. The company takes ownership of customer success, going far beyond cryo-TEMs to span the entire workflow (http://www.thermofisher.com/pharmadrugdiscovery/), with significant gene-to-drug expertise that can be used by customers to accelerate their platform and application development. Lastly, the company has an extensive network of key opinion leaders in protein science who bring expert understanding of highly complex drug target classes and are a hotbed for the inception of new therapeutic opportunities.

Figure 3. Based on our extensive expertise in helping customers develop cryo-EM discovery platforms, Thermo Scientific cryo-TEMs can help accelerate the discovery of medicines for the most important diseases. Applications range from membrane proteins to epitope mapping, vaccines, degraders, chimeric antigen receptor T (CAR T) cells, off-target effects and more.
Taken together, this intersection of transformative structure-based drug design technologies and drug target excellence provides fertile grounds for better medicines that venture capitalist firms like Third Rock Ventures are betting on. Companies like Septerna, Inc., Therapeutics may be the precursors of a bigger cryo-EM startup wave.
For research use only. Not for use in diagnostic procedures. For current certifications, visit thermofisher.com/certifications © 2022 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.
References:
- Wigge C, Stefanovic A, Radjainia M. (2020) The rapidly evolving role of cryo-EM in drug design. Drug Discov Today Technol 38:91–102.
- Congreve M, de Graaf C, Swain NA, Tate CG. (2020) Impact of GPCR structures on drug discovery. Cell 181(1):81–91.
- Brown AJH, Bradley SJ, Marshall FH et al. (2021) From structure to clinic: Design of a muscarinic M1 receptor agonist with potential to treatment of Alzheimer’s disease. Cell 184(24):5886–5901.e22.
- Draper-Joyce CJ, Bhola R, Wang J et al. (2021) Positive allosteric mechanisms of adenosine A1 receptor-mediated analgesia. Nature 597(7877):571–576. doi: 10.1038/s41586-021-03897-2. Epub 2021 Sep 8.
- Frye L, Bhat S, Akinsanya K, Abel R. (2021) From computer-aided drug discovery to computer-driven drug discovery. Drug Discov Today Technol 39:111–117.
- Zhang X, Belousoff MJ, Zhao P et al. (2020) Differential GLP-1R binding and activation by peptide and non-peptide agonists. Mol Cell 80(3):485–500.e7.
- Zhang X, Belousoff MJ, Liang YL et al. (2021) Structure and dynamics of semaglutide- and taspoglutide-bound GLP-1R-Gs complexes. Cell Rep 2021 36(2):109374.
- Nakane T, Kotecha A, Sente A et al. (2020) Single-particle cryo-EM at atomic resolution. Nature 587(7832):152–156.