The Path to Productivity: Tackling the Crisis in Drug Development using Improved Preclinical Models
“The more positive anyone is about the past several decades of progress [in pharmaceutical development], the more negative they should be about the strength of countervailing forces.” These foreboding words were penned in a seminal 2012 article by Jack Scannell, author of Eroom’s Law, to illuminate the drug development industry’s productivity crisis.
Productivity measures how efficient drug development is, often presented as the number of drugs that can be brought to market given a set amount of effort or investment. Consider pharmaceutical development in the 1950s: Data presented by Scannell and his co-authors showed that, with the contemporary equivalent of $1 billion US dollars, the industry was able to produce around 30 new drugs. In contrast, that same investment in 2023 would not even produce one new therapeutic (see Figure 1).
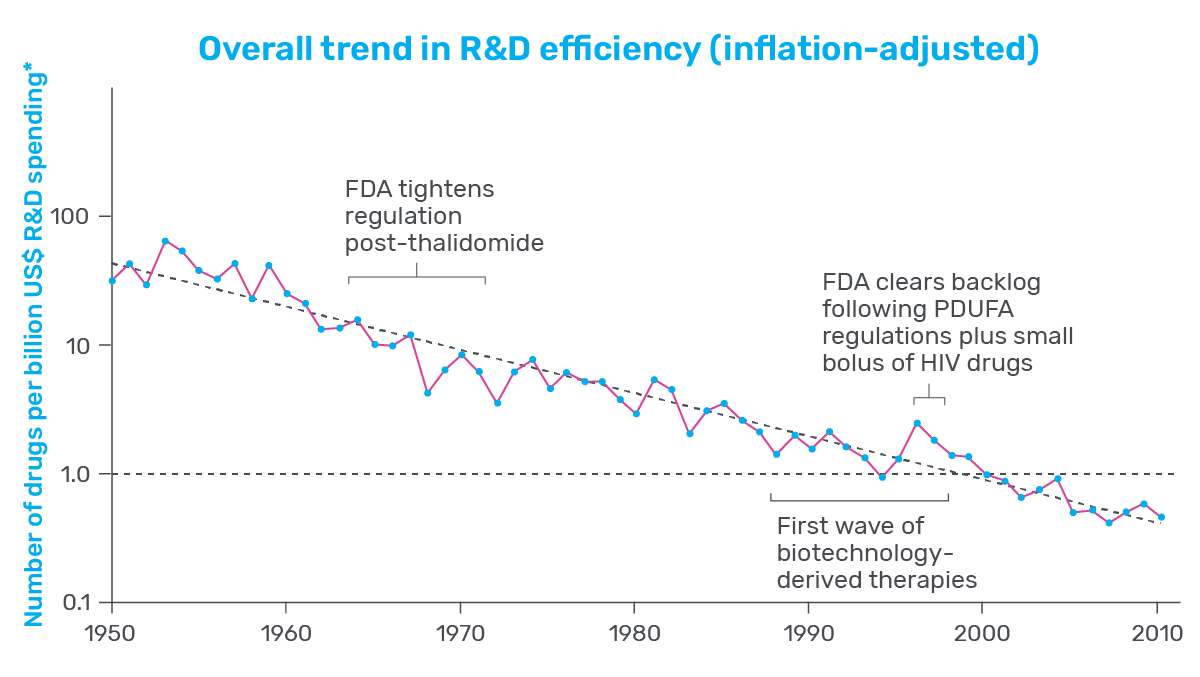
Figure 1: Graph showing the change R&D efficiency since 1950.
This decline in productivity has many in the industry concerned, as lower productivity means steeper development costs and slower progress. As a result, drug prices increase for patients, who are left desperately waiting for therapeutic relief.

All of the news, delivered with full-text to your inbox. For professionals discovering, developing, and marketing biopharmaceutical drugs.
Scannell and many others have worked to diagnose the various factors behind the current productivity crisis—the “countervailing forces.” Through their efforts, many potential causes have been identified, one of which stands out as profoundly impactful: improving the accuracy of preclinical models.
Preclinical Drug Development
Preclinical drug development is highly speculative: Researchers must foretell how compounds will behave in the human body and identify the few that are safe and therapeutic. To do this, they use models as proxies for the human body, and none serve a more prominent role than non-human animal models.
Rodents, primates, and other non-human animal models have long been the gold standard in preclinical toxicology screening. With complex and interconnected tissues, animals allow researchers to test the effect of their drug in a dynamic system resembling the human body. As such, animals are the last filter in the drug development process, tasked with weeding out toxic drugs before clinical trials.
Despite the ubiquity of animal models, ample evidence indicates they are far from perfect. Approximately 90% of drugs entering clinical trials fail, with roughly 30% of those failures attributed to unforeseen toxicity. This indicates that, at minimum, animal models alone are insufficient decision-making tools—too often, they get it wrong, and both patients and drug developers pay the price.
This failure is central to the current productivity crisis. Though researchers now have access to next-generation sequencing, combinatorial chemistry, and automation, drug development costs have increased nearly 80-fold since 1950 to a staggering $2.3B per approved drug. And approximately 75% of these costs can be attributed to failure.
It stands to reason that reducing clinical trial failure rates will improve the efficiency of drug development. Not only are failed trials expensive, but they also take up clinical resources that could be used to advance successful drugs. Since clinical trial failure rates reflect the quality of drugs that enter trials, improving this quality should increase industry productivity.
How should researchers go about doing this? Revisiting his work a decade later, Scannell provided powerful guidance: The quality of the compounds that enter clinical trials is a consequence of the preclinical models used to select them, and even small improvement in the quality of the preclinical models—more specifically, their predictive validity—can have a substantial impact on productivity. Enter more human-relevant preclinical models like the Liver-Chip.
Improving Productivity with Organ-Chips
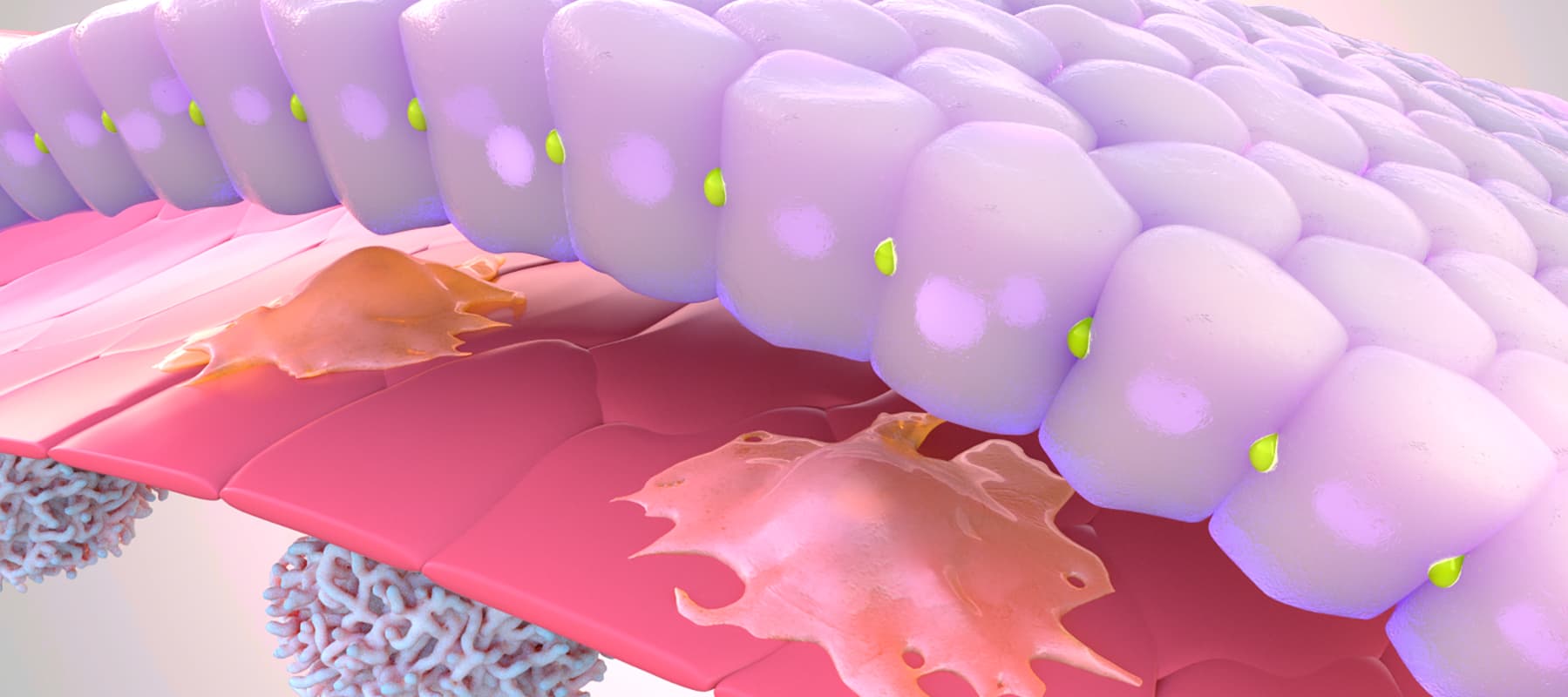
In a recently published study, Emulate scientists showed that the Liver-Chip—a specialized Organ-Chip that mimics the human liver—can identify compounds’ potential to cause drug-induced liver injury (DILI) far more accurately than traditional in vitro and animal models.
Briefly, Organ-Chips are three-dimensional culture systems that combine heterogeneous cell culture, fluid flow, and several features of the tissue microenvironment to mimic human organ function in an in vitro setting. Evidence indicates that human cells cultured in Organ-Chips behave remarkably similar to their in vivo counterparts. Among many promising applications, these chips are particularly well suited for preclinical toxicology screening.
In their study, the Emulate researchers found the Liver-Chip to be a highly sensitive and specific tool for detecting hepatotoxic compounds. In particular, the Liver-Chip showed a sensitivity of 87% and specificity of 100% against a series of drugs that had progressed into the clinic after being tested in animal models, only to later be revealed as toxic when given to patients. Therefore, these drugs well represent the current gap in preclinical toxicology testing, through which some hepatotoxic drug candidates evade detection and advance into clinical trials.
If the Liver-Chip can fill the gap left by animal models, Scannell’s framework suggests that it could profoundly affect the industry’s productivity by reducing the number of safety-related clinical trial failures.
To calculate how this reduction may impact industry productivity, Emulate researchers teamed up with Jack Scannell to build an economic value model. They showed that applying the Liver-Chip in all small-molecule drug development programs could generate $3 billion dollars annually for the industry through improved productivity. This is approximately $150M per top pharmaceutical company. And, that’s just for the Liver-Chip. In addition to hepatotoxicity, cardiovascular, neurological, immunological, and gastrointestinal toxicities are among the most common reasons clinical trials fail. If Organ-Chips can reduce these clinical trial failures with a similar 87% sensitivity, the resulting uplift in productivity could generate $24 billion for the industry annually—roughly $750M to $1B per top pharmaceutical company.
Even when accounting for the cost of integrating and running Liver-Chip experiments, the savings from reducing clinical trial failures are substantial. Moreover, the extra bandwidth would permit advancing more promising compounds. The Emulate researchers’ work demonstrates that improving productivity in drug development is possible, and it starts with developing better models. As the industry embraces the potential of Organ-on-a-Chip technology and continues to explore its application in various areas of drug development, there is hope for a future with improved productivity and faster delivery of life-saving therapeutics.