
Transport Simulation Testing for Your Therapy is the Best Way to Assure FDA Expedited Program Approval
Modality Solutions is an ISO:9001-registered biopharmaceutical cold chain engineering firm with unique transport simulation capabilities that support accelerated regulatory approval for biologics and advanced therapeutic medicinal products (ATMP). Our expertise combines traditional validation engineering approaches with regulatory knowledge into a methodology tailored for the life sciences industry. We provide insight and execution for the challenges faced in your cold chain logistics network.
Transport Simulation Supports Approval Timelines in Expedited Programs
Your cold chain process validation is one component considered in the FDA expedited program submission and approval process. A robust, risk-based quality assessment of a drug product requires shipping studies to ensure chemical and physical stability of your therapy during transport¹. Expedited programs are intended to speed up the availability of new therapies. This flexibility can compress approximately eight years of traditional development and validation activities into roughly 18 months. Each clinical phase is condensed, and validation data are reviewed on a rolling submission basis to follow an accelerated timeline.
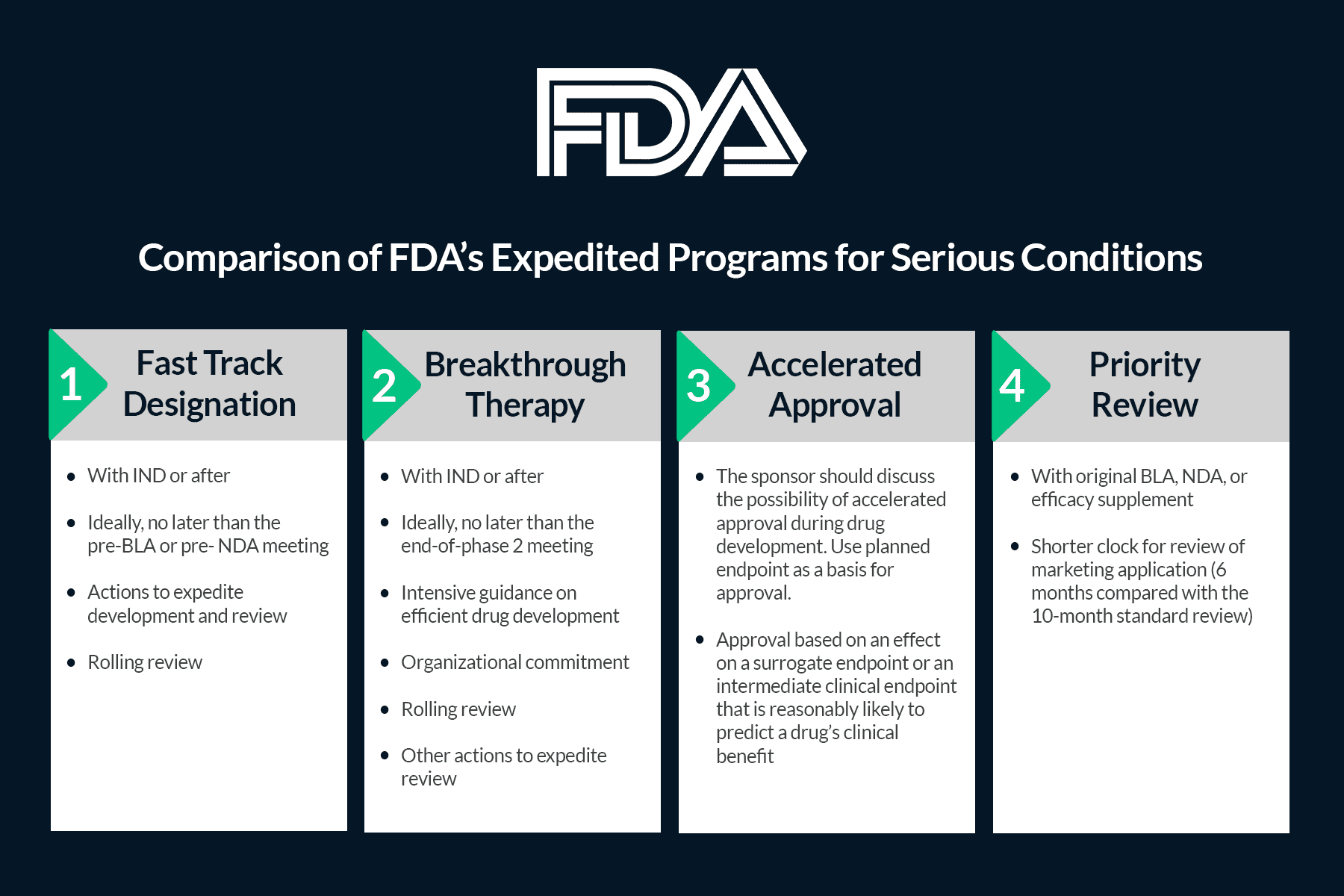
Transport simulation validates your cold chain supply chain in a cost-effective, reliable, and expeditious way to keep your development program on an accelerated approval timeline. More importantly, transport simulation challenges drug product physical and chemical stability in a way that real-world shipping studies can never do.
Transport simulation testing requires only three days to test your therapy for most multimodal transport lanes from the point of manufacture to the wholesaler, specialty pharmacy, or direct to patient delivery models. The data provided by the simulation testing, coupled with post-testing analytical results are robust and reliable.
Most importantly, this approach gives you something real-world shipping studies can never guarantee – testing at the edges of your operating space for all five major environmental hazards in the cold chain. Transport simulation provides a clear picture of whether the worst-case conditions affect your drug formulation’s physical and chemical integrity based on the drug formulation’s stability profile.

Real-Time Concurrent Hazard Testing Assures Validating Across the Entire Operating Space
The only way to appropriately challenge your therapy is by using concurrent environmental hazards with non-accelerated profiles that simulate seasonal conditions. Transport simulation testing is the only reliable way to evaluate drug product quality with testing at the edges of your operating space with worst-case exposures to the five major concurrent transport environmental hazards. Real-world shipping studies alone cannot guarantee that these hazards will stress your drug product therapy at all, let alone all hazards at worst-case intensity concurrently.²
Transport simulation is able to predict drug product stability by exposing your therapy to the hazards of distribution (such as temperature, pressure, shock, vibration, and humidity) across the entire operating space, the quality of the drug product for all transport modes and seasonal variations. Early drug product transport simulation testing allows sponsors to make changes to formulations, if necessary, and create a more stable drug product with less sensitivity to environmental hazards.
Real-world shipments cannot guarantee testing across the entire operating space, cannot be assured proper control of testing parameters, and cannot be used afterward for root cause analysis or optimization if drug product formulation stability issues are uncovered. Having reliable and repeatable transport simulation technology and testing techniques will maximize your chances of regulatory approval and ensure your timeline commitments.
Transport Simulation Testing Has Been Accepted by Regulators for Over a Decade
Transport simulation was first used in submissions in the early 2000s to show drug product robustness during transport for monoclonal antibodies. Early simulation testing only exposed drug products for seasonal temperature ranges and not atypical highs and lows, multimodal transport vibration, and shock events were tested separately. Since the early use of transport simulation, the technology has improved to include concurrent testing and added humidity and atmospheric pressure testing as well.
Simulation technology has gotten better and is in widespread use across the biopharmaceutical industry. Modality Solutions has a proprietary transport simulation laboratory with the unique ability to simultaneously test any drug product formulation for five environmental hazards commonly affecting protein-based formulations: temperature, shock, vibration, humidity, and pressure. We have been using transport simulation testing to facilitate over 70 successful regulatory submissions over the last seven years.
More than 30 countries have adopted or amended Good Distribution Practices for pharmaceuticals in the past decade.³ While the specifics vary, they all advise some degree of environmental or condition testing, monitoring, and tracking to ensure therapeutics and diagnostics are handled in a way that ensures their safety and efficacy.
U.S. Pharmacopeia Good Storage and Distribution Practices for Drug Products Chapter 1079, advises operational and performance testing for products, as well as temperature profiling studies for transportation and warehouses.⁴ ASTM D4169, “Standard Practices for Performance Testing of Shipping Containers and Systems” addresses packaging durability for each shipping mode.⁵
Simultaneous Testing of All Seasonal Variations and Transport Modes is Possible All Year-Round
Expedited program approval timelines and rolling submissions accelerate the need for timely assessment of your proposed shipment lanes with prompt validation data. Seasonal transport validation for your cold chain logistics network is not available year-round with real-world shipping studies alone. Transport simulation allows all seasonal and transport modes (i.e., air, ground, and ocean) to be conducted across 3-4 days of testing and generate results to include in your submission.
We recommend conducting a risk assessment followed by compiling a Validation Master Plan that documents overall validation strategies for the lifecycle of your product including your approach to seasonal variations. Before testing, the risk assessment can identify which transport environmental hazards place the therapy at the highest risk and allows the focus on the simulation testing on the transportation environmental hazards (e.g., hot or cold, shock, vibration, pressure, or humidity) most relevant to your drug product quality.
Relying on real-world shipping studies alone can delay or force an incomplete submission to regulators because seasonal variation studies can only be conducted in tight windows with no guarantee of success. Planning a real-world shipping study and waiting for the “dog days” does not mean that the expected extreme temperatures will be seen during your designated testing period. Only transport simulation testing can guarantee that the coldest temperatures of winter and the highest temperatures of summer can be experienced in a single protocol in a 3 to 4-day testing cycle.
¹ https://www.fda.gov/files/drugs/published/Expedited-Programs-for-Serious-Conditions-Drugs-and-Biologics.pdf
² Fleischman, M. et al., “Shipping-Induced Aggregation in Therapeutic Antibodies: Utilization of a Scale-Down Model to Assess Degradation in Monoclonal Antibodies,” Journal of Pharmaceutical Sciences, Volume 106, Issue 4, pp.994-1000, April 1, 2017.
³ A Global Review of Good Distribution Practices, IQPC, https://www.iqpc.com/media/8378/34072.pdf
⁴ U.S. Pharmacopeia Good Storage and Distribution Practices for Drug Products, chapter 1079, http://www.pharmacopeia.cn/v29240/usp29nf24s0_c1079.html
⁵ Standard Practice for Performance Testing of Shipping Containers and Systems. ASTM, https://www.document-center.com/standards/show/ASTM-D4169
Gary Hutchinson is President of Modality Solutions, a biopharmaceutical cold chain engineering firm specializing in global cold chain validation. He has designed life sciences supply chain logistics and biopharmaceutical cold chains for the last 20 years. He is a member of the Forbes Technology Council, speaker for various pharmaceutical conferences and has successfully developed cold chain transport validation data for more than 50 new drug products with multiple regulatory agencies around the globe in the last 5 years.