Why it Works: Manufacturing a Vaccine in a Multi-Product Facility.
COVID-19 launched the pharmaceutical industry to the frontline in the battle against the fast-spreading global pandemic. The goal: distribute a safe, effective vaccine as quickly as possible. Major players in the vaccine market needed to partner with contract development and manufacturing organizations (CDMOs) to achieve the goal of mass vaccine quantities under expedited timelines. With CDMOs stepping up to play a critical role in the vaccine manufacturing process, multi-product CDMO facilities took the spotlight. Partnerships quickly formed as the race to save lives and fight a pandemic was on.
In 2020, the U.S. Department of Health and Human Services and the U.S. Department of Defense called upon CDMOs to support the expansion of the United States’ capacity for manufacturing and distributing vaccines or therapeutics related to the COVID-19 pandemic. One example is through a public-private partnership where the federal government reserved fill/finish capacity on a CDMO’s commercial filling line for use by federal partners that are developing COVID-19 vaccines or therapeutic treatments. That company’s capacity not only supported the U.S. government’s Operation Warp Speed efforts and the COVID-19 pandemic response but also supports an increase in U.S. preparedness for future public health emergencies.
Now more than ever flexibility is key to success in the pharmaceutical manufacturing industry, specifically for CDMOs that serve a variety of clients and products. Combine heightened awareness with elevated demand and reduced timelines, and the demand of CDMO capacity, quality, and modern equipment have increased exponentially since the start of 2020. CDMOs must be equipped to operate in a fast-paced environment without sacrificing quality. The pharmaceutical manufacturing industry is consistently challenged to be more cost-effective and to reduce timelines to get products to market faster. Organizations that can meet these demands and uphold strong relationships with partners and vendors will find success.

All of the news, delivered with full-text to your inbox. For professionals discovering, developing, and marketing biopharmaceutical drugs.
Though taking on additional accelerated production while meeting existing contracts may sound daunting, a well-equipped CDMO prepared with the right technology and team can competently and effectively rise to the challenge. Advanced technology, flexible equipment and agile team members are defining factors needed for those selected for the mass manufacture of a vaccine, under time-sensitive circumstances, and in facilities already producing multiple products. The following factors are vital in an ever-changing landscape.
Adaptability is Key
Meeting the demands of multiple clients, products, and batch sizes takes incredible flexibility. When considering the biggest asset to increased flexibility or capabilities, building a new state-of-the-art facility or expanding upon current facilities likely comes to mind.
When deciding on facility investments, it is vital to consider the potential obstacles of retrofitted facilities and technology. Retrofitted and outdated facilities make it difficult to establish one-way product flow, impeding their flexibility and adaptability. Utilities and interfaces must be prepared to meet the demands of the latest pharmaceutical processes. Requirements range in complexity from the regulation of varying air pressure capacities to ensure aseptic processing, to the HVAC capabilities needed to maintain a sterile environment. Air-handling systems and flows must be designed to allow for the capture of airborne particles. Newer facilities can be designed as such, with the appropriate people and product flows to ensure product containment throughout the sterile manufacturing process.
Key players in the CDMO space and essential partners in producing life-saving vaccines saw an influx in expansions due to the dramatic increase in demand for vaccines and therapeutics. One example being Grand River Aseptic Manufacturing (GRAM) who embarked on a phase 2 expansion of their large-scale facility a year after opening the facility’s doors, plus recently completing their new finishing center in under one year.
Modern, state-of-the-art fill/finish facilities provide access to top-quality sterile injectable drug manufacturing for all batch sizes while cutting down the variables for cross-contamination and needless waste. Multi-product risk assessments create less room for error and reduces variables of contamination. CDMOs should provide a multi-product risk assessment for each facility, identifying gates for potential cross contamination and making sure the CDMO has procedural or engineering controls in place to mitigate the potential risks. Efficient processes and facility design support the production of life-saving vaccines or therapeutics.
FDA and EU guidance and regulatory requirements encourage the industry to modernize and keep up with the latest safety and quality control technology. CDMOs not modernizing their processes or facilities may run the risk of heavy scrutiny during audits and find themselves subject to the associated consequences. Despite these risks, an entirely new facility or expanded space isn’t always attainable. This is especially true when updates are not a viable solution due to budget, space, or time restrictions. Innovative and up-to-date facilities are essential for future growth and emergency preparedness.

The Power of Flexible Technology
Modern equipment and technology allow for greater flexibility, product containment, and capabilities. Leaning on regulatory guidelines, as well as experienced contractors and original equipment manufacturers (OEMs), CDMOs can confidently choose the solution that best fits current and future client needs.
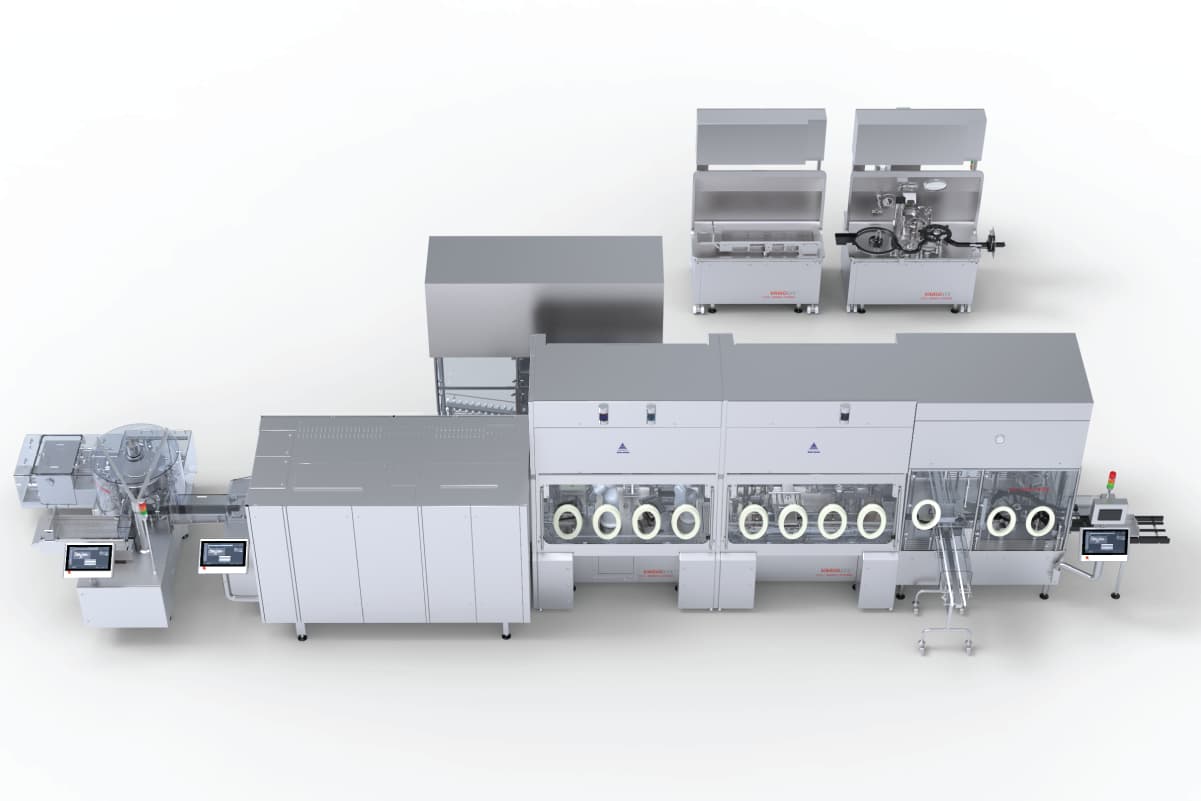
One way to achieve this is to invest in versatile technology like the VarioSys syringe and vial filler with SKAN isolator. The modular VaioSys technology offers unique, flexible L-flange technology. The L-flange houses the filling system that fits into the isolator. Because the L-flange can be replaced with a new piece of filling technology, it allows for the use of a vial filler, syringe filler, and aseptic formulation isolator—all utilizing one room and one isolator.
The unique L-shaped flange enables a quick exchange of precisely tailored modules, ensuring a universal sterile working environment for aseptic, or highly active pharmaceutical products. This flexibility offers a huge advantage when it comes to selecting a multi-product facility. A customer who has multiple products in their portfolio with many different presentations, ranging from vial to syringe or cartridge presentation, would be able to fill different configurations in one clean room.
Strategic updates in technology are what CDMOs must strive for to efficiently increase future capacity, deliver consistently to clients and uphold regulatory guidelines.
The Value of Experience
Increasing flexibility and capacity with innovative technology is one thing, but it means very little without the crucial support of talented and agile team members. Adaptability within your team is essential to success, and just as critical is their expertise. Every operator and MS&T (Manufacturing, Science and Technology) specialist must be an expert in the equipment on hand to keep production running smoothly in a multi-product facility. Investing in multiple pieces of equipment and technology from the same OEM helps staff understand all aspects of the technology more efficiently. Team members can transfer the same level of expertise from machine to machine, cutting down on training and troubleshooting between batches and expediting production for clients. It also creates operators that are incredibly skilled when going from one machine to another and transitioning between product batches.
In addition to expert staff, quality relationships with OEMs provide a multitude of mutual benefits. Strong relationships have proven to reduce timelines and adapt new support methods under situations when getting products to market quickly under expedited timelines is critical. Strength in relationships has exponentially come to light while the industry has battled the COVID-19 pandemic. Consistent technology coupled with skilled operators makes for a safe and efficient multi-product CDMO.
Knowledgeable team members backed by supportive partners is vital to the success of projects, especially those with critical deadlines. While CDMOs must invest in sophisticated technology to enforce regulatory guidelines, dedicating resources to the curation of a skilled team is crucial for the company’s success and the job market in the local community. Doing so improves the ability to meet new client demand as equipment, facilities, and industry expectations continue to expand.

Looking Ahead
The success or failure of CDMOs to adapt to times of crisis and unparalleled demand will ultimately depend on their ability to stay nimble. Looking towards the future, anticipating regulatory requirements, and understanding the latest equipment and technology to support the varied needs of current and prospective clients is imperative. Advanced digital solutions for monitoring and tracking products and processes will continue improving to support containment in multi-product facilities. Modular equipment designs of the future will allow for the benefits of segregation without the expense of separate fill lines, adding flexibility and technology for easy scale-up.
Expectations on these facilities and vaccine development will stay at the forefront for years to come. The battle against a pandemic and race to a vaccine has ignited some of the most unlikely relationships including those with the federal government, global pharmaceutical companies, and sterile injectable CDMOs. The spirit of collaboration has never been stronger, and the industry is better off for it. Emergency preparedness and support that ensures the pharmaceutical industry is ready to accelerate timelines when the need arises has taken a front row seat in vaccine manufacturing, and CDMOs are ready for the challenge.