
Will Communicating with Personal HCPs Get the Industry Closer to More Diverse and Fully Enrolled Clinical Trials?
In 2021, 50 novel medications were approved by the Federal Drug Administration (FDA), the third-highest number of approvals on record and one of many indicators of the extraordinary advancements the medical field has seen in recent decades. But progress within the industry is not equally distributed. When acknowledging the contributions of more than 38,000 patients in its Drug Trials Snapshot of the same year, the FDA noted that “there were many programs where representation from certain racial and ethnic groups was low.” This carefully worded observation describes a long-standing limitation in the clinical trial sector.
People of color make up just one-fifth of clinical trial participants in the US. That is far less than the non-white US population reported in the most recent census data and does not address other critical variables that can influence healthcare outcomes, such as age, gender and socio-economic factors.
In April of 2022, the FDA issued its 9th guidance to combat the negative impacts of demographically-biased trials. But how can the industry ensure that this latest guidance achieves the results that all involved want and need? In short: what can we do that has not already been done for more fully enrolled – and equitable – clinical trials?
Leveraging the patient journey
To answer that question, we must pay attention to the factors at the core of every clinical trial: patients and caregivers. A person’s decision to participate in a clinical trial is rarely bound to empirical data. Rather, patients respond most positively to personal, individualized communications to guide their choices, such as conversations with their doctors. However, these conversations between healthcare professionals (HCPs) and their patients aren’t happening frequently enough. 90% of patients worldwide indicate that their doctors have never discussed a clinical trial with them. Recent research published by the Center for Info & Study on Clinical Research Participation (CISCRP) revealed that only 14% of potential volunteers learned about a study from their HCP.
This raises an important question that challenges the status-quo of clinical trial recruitment: could communicating with patients’ personal HCPs about relevant clinical trials tip the scales in favor of more equitable, diverse and fully enrolled trials? And if so, could they reach more diverse patient populations and help bridge the gap between patients who mistrust in the pharma industry and potentially life-saving clinical trials?
Yes, HCPs are willing to refer
To understand if personal HCPs are willing to discuss clinical trials with their patients – and, if so, how best to reach out to them – Syneos Health queried 151 doctors across five different specialties and sizes of practice on their viewpoints on clinical trials. The results were emphatically positive: 93% of doctors would refer patients to clinical trials (Figure 1).
Figure 1: Surveyed HCPs were willing to refer patients to clinical trials
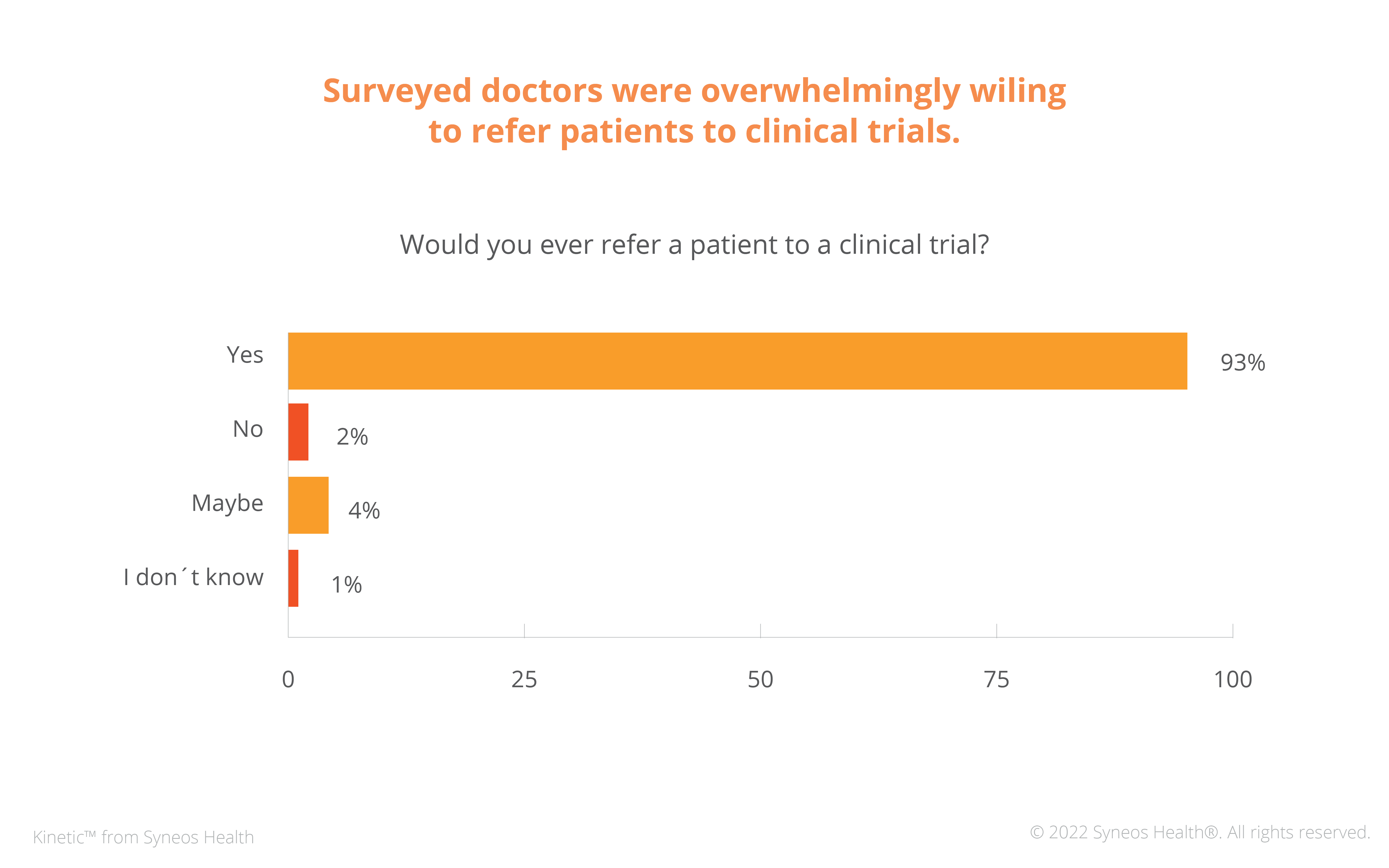
Results also suggest that most doctors who hadn’t addressed clinical trials with their patients did so due to a lack of information. 67% of them learned about trials only every few or once every six months. In contrast, a majority of those who had discussed clinical trials with their patients about trials (58%) learned about trials at least once a month.
Overall, data suggests that for various reasons, simply educating more HCPs outside of standard site referral networks about trials could be enough to increase trial enrollment via referrals.
Challenging traditional site outreach models
Informing HCPs of clinical trials is not new, but its conventional application means it falls short of its potential. Traditional recruitment outreach identifies HCPs within a site’s referral network and/or by specialty. Important factors such as patient volume or whether an HCP is treating trial-relevant patients are either not considered consistently across the full cohort of potential referrers. As a result, significant potential referrers never learn about trials that might benefit their patients. Static referral networks may even compound unintentional bias in favor of the prevailing demographic of a site’s patient cohort, omitting entire groups crucial to diverse enrollment (Figure 2). But we don’t often consider these variables when strategizing site selection or site outreach methodology.
Figure 2: HCP outreach models
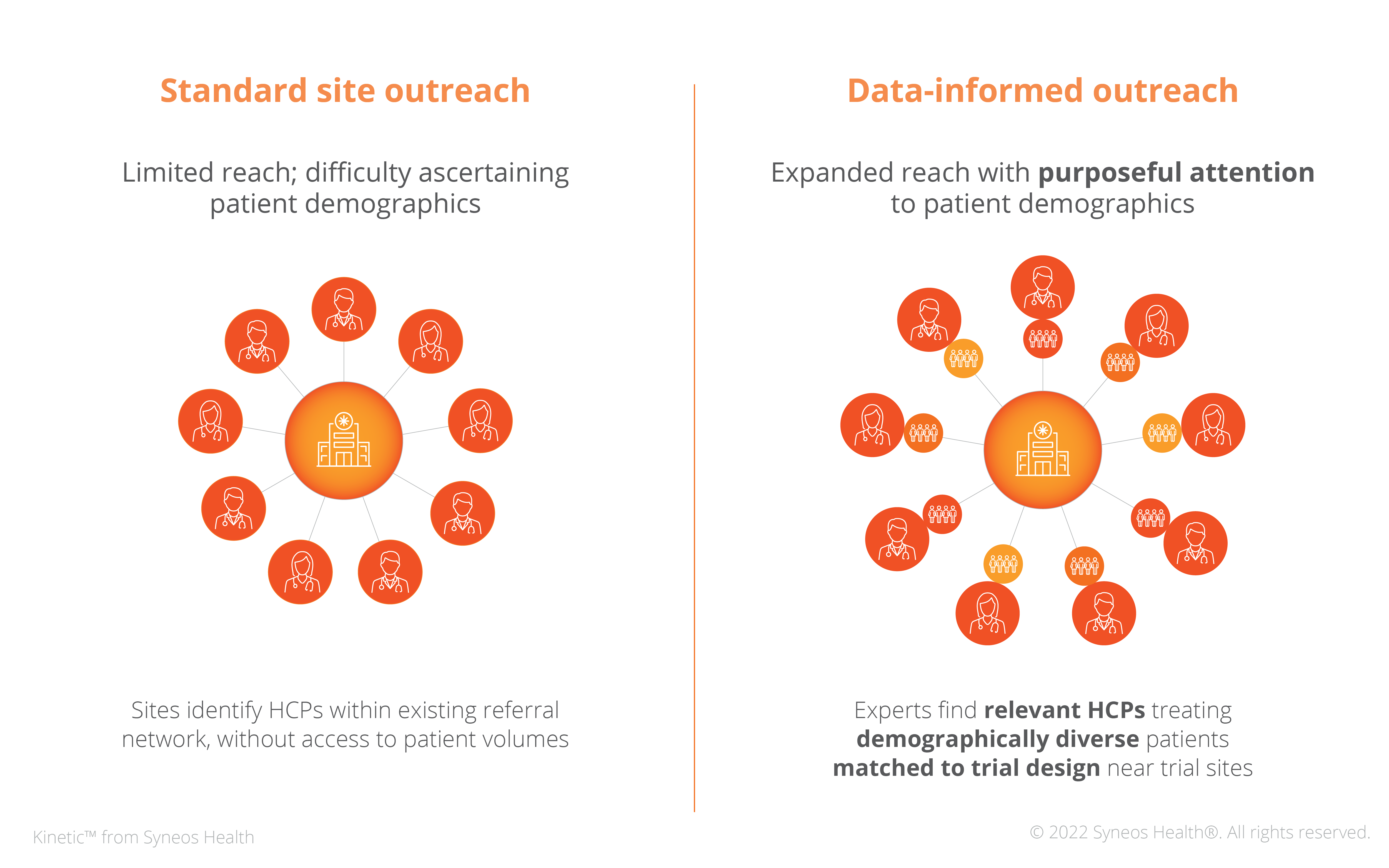
These historic limitations do not suggest any failure on the part of study sites. Nor do they uncover a lapse of attentiveness on the part of recruitment teams. Until now, there has not been a consistent, workable means to identify and locate HCPs who practice outside a site’s referral network. Traditional outreach is dependent on some level of active and time-consuming participation on the part of an HCP. This need for proactive action is a significant drawback for often overburdened providers and clinic staff.
Artificial intelligence (AI)-powered and data-driven technologies can help overcome these limitations. Digital messaging campaigns powered by these advances and targeted at precise HCPs can encourage clinical trial referral. Leveraging the vital patient/provider relationship that we know influences a patient’s clinical trial participation decision-making process in the service of more full clinical trial enrollment, these campaigns can also empower more equitable enrollment.
These technologies enable clinical trial teams to identify and engage HCPs outside a site’s referral network. These providers are most likely to be helping patients navigate critical decisions in their healthcare journeys relevant to a specific trial and the HCPs who treat patients with diverse backgrounds. An in-depth, hyper-targeted, personalized outreach can empower underrepresented demographics to consider trials through engaging their HCPs. This purposeful attention to patient access and demographics supports more inclusive and equitable clinical trial enrollment.
Contributions by Syneos Health
Our industry needs new channels to advance the recruitment of diverse and epidemiologically relevant patient populations, while balancing data privacy. Syneos Health innovations in technology, data and behavioral science provide immediately actionable solutions for this long-standing industry challenge. Our team has the ability to connect the data, control its access, and stay in strict compliance with privacy regulations. We are carrying forward the momentum for change, offering a suite of tech-enabled diversity solutions enabling you to take action on FDA guidelines, industry commitments and the ethical responsibility to communities for diverse and equitable clinical trials.
Syneos Health Authors:
 Monika Hirschbichler
Monika Hirschbichler Vice President,
Program Strategy
 Brooke Belk
Brooke Belk Vice President,
Program Strategy
 Melissa Lepore
Melissa Lepore Associate Director,
Program Strategy