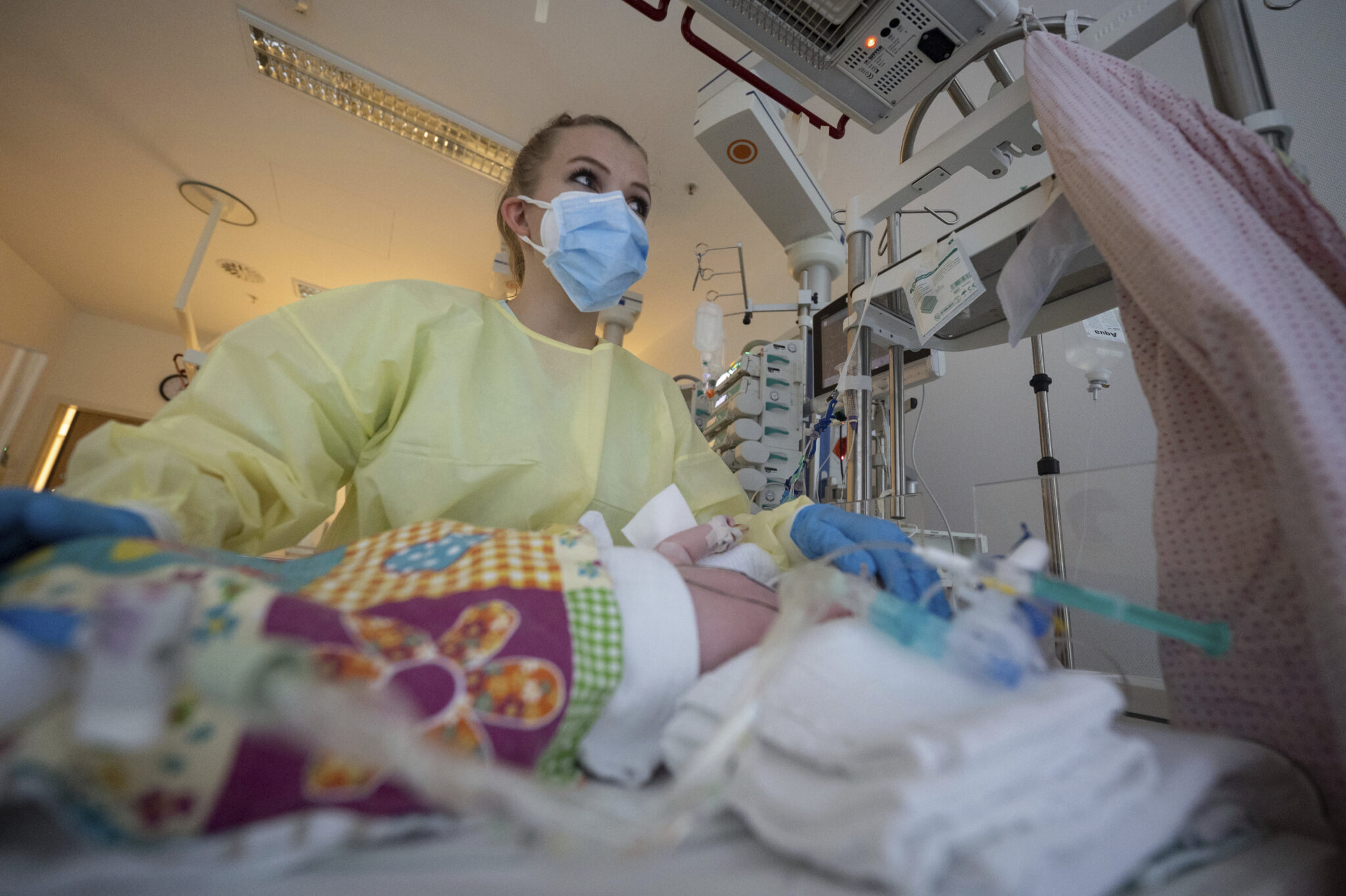
An intensive care nurse treating a newborn with RSV in Germany. Sanofi’s new antibody is designed to protect all infants from the common and occasionally severe infection. (Marijan Murat/picture-alliance/dpa/AP Images)
With new RSV antibody, Sanofi looks to avoid a repeat of a 20-year-old pricing controversy
Twenty-four years ago, the FDA approved a drug as controversial as it was innovative.
Known as Synagis, it was OK’d for protecting certain infants against …
Sign up to read this article for free.
Get free access to a limited number of articles, plus choose newsletters to get straight to your inbox.